Journal of Heredity
Transcription
Journal of Heredity
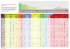
Journal of Heredity Advance Access published March 3, 2010 ! The American Genetic Association. 2010. All rights reserved. For permissions, please email: [email protected]. Journal of Heredity doi:10.1093/jhered/esq017 Experiments with Digital Organisms on the Origin and Maintenance of Sex in Changing Environments DUSAN MISEVIC , CHARLES OFRIA, AND RICHARD E. LENSKI Address correspondence to Dusan Misevic at the address above, or e-mail: [email protected]. Abstract Many theories have been proposed to explain the evolution of sex, but the question remains unsettled owing to a paucity of compelling empirical tests. The crux of the problem is to understand the prevalence of sexual reproduction in the natural world, despite obvious costs relative to asexual reproduction. Here we perform experiments with digital organisms (evolving computer programs) to test the hypothesis that sexual reproduction is advantageous in changing environments. We varied the frequency and magnitude of environmental change, while the digital organisms could evolve their mode of reproduction as well as the traits affecting their fitness (reproductive rate) under the various conditions. Sex became the dominant mode of reproduction only when the environment changed rapidly and substantially (with particular functions changing from maladaptive to adaptive and vice versa). Even under these conditions, it was easier to maintain sexual reproduction than for sex to invade a formerly asexual population, although sometimes sex did invade and spread despite the obstacles to becoming established. Several diverse properties of the ancestral genomes, including epistasis and modularity, had no effect on the subsequent evolution of reproductive mode. Our study provides some limited support for the importance of changing environments to the evolution of sex, while also reinforcing the difficulty of evolving and maintaining sexual reproduction. Key words: Avida, digital evolution, epistasis, evolution of sex, genetic architecture, modularity Why sex? Sexual reproduction is costly and complicated, yet it is widespread in the biological world (Bell 1982). This paradox has long fascinated biologists and generated a multitude of hypotheses and experimental tests (Weismann 1889; Bell 1982; Michod and Levin 1988; Kondrashov 1993; Rice 2002). Perhaps the simplest and most intuitive explanation is that sex, by increasing variation, can accelerate the rate of adaptation to novel or changing environments (McPhee and Robertson 1970; Malmberg 1977; Goddard et al. 2005; Cooper 2007). Theoretical analyses have suggested that sex will be favored especially when the fitness contributions of particular allele combinations frequently switch between being advantageous and disadvantageous (Charlesworth 1976, 1993b; Maynard Smith 1978). Such reversals in selection can be caused by host–parasite interactions or other forms of coevolution (Van Valen 1973; Hamilton 1980; Lively 1987; Peters and Lively 1999; Lively and Dybdahl 2000; Salathe et al. 2008). However, the more general theoretical requirement is for changing environments, regardless of the precise ecological basis (Sasaki and Iwasa 1987; Charlesworth 1993a; Barton 1995; Kondrashov and Yampolsky 1996; Otto and Michalakis 1998; Waxman and Peck 1999; Otto and Nuismer 2004; Gandon and Otto 2007). We note that selection caused by parasites typically acts in a frequency- or density-dependent manner, whereas stochastic changes in the environment—even those that reverse the direction of selection on particular traits—would not necessarily act in the same way. Also, even if sexual reproduction can accelerate adaptation to changing environments, such an effect might be more important in maintaining sex than in facilitating its origin. That is, this 1 Downloaded from http://jhered.oxfordjournals.org at Peking University on August 19, 2010 From the Program in Ecology, Evolutionary Biology, and Behavior, Michigan State University, 103 Giltner Hall, East Lansing, MI 48824 (Misevic, Ofria, and Lenski); the Department of Computer Science and Engineering, Michigan State University, 3115 Engineering Building, East Lansing, MI 48824 (Ofria); and the Department of Microbiology and Molecular Genetics, Michigan State University, 2215 Biomedical Physical Sciences, East Lansing, MI 48824 (Lenski). Dusan Misevic is now at the INSERM U1001, Centre de Recherches Interdisciplinaires (CRI), Faculté de Médecine, site Cochin Port-Royal, Université Paris Descartes, 24 rue du Faubourg Saint Jacques, 75014 Paris, France. Journal of Heredity Methods Experimental System All experiments were conducted using Avida software (available without cost at http://avida.devosoft.org/) with the default settings, unless otherwise indicated. Digital organisms in Avida are short self-replicating computer programs that can reproduce either asexually or sexually, depending on which divide instruction they execute. Digital genomes are built from the default instruction set with 27 instructions including 2 divide instructions, divide-sex and divide-asex, only one of which can be expressed by any individual (Misevic et al. 2006). Mutations may affect the organisms’ reproductive mode as well as their interactions with substrates present in the environment. In this study, point, insertion, and deletion mutations occurred at rates of 0.002, 0.0005, and 0.0005 per instruction copied, respectively, with the same mutation rates applied to the divide instructions as all others; these are the default rates in Avida, and they were used for consistency with previous studies (Wilke et al. 2001; Ofria and Wilke 2004). The carrying capacity (maximum population size) was 3600 organisms; when a population was at carrying capacity, each new offspring replaced a randomly chosen organism from anywhere in the population. For the first 1000 updates of each experimental run, the populations evolved in the same constant environment with 9 substrates used previously (Misevic et al. 2006) to evolve the organisms that served as the ancestors in this study, after which additional and changing substrates were introduced, as described below in the section on Digital Metabolism. An update is a unit of time in Avida during which an average of 30 instructions are executed per organism in the population. A generation typically requires 5–10 updates, with the precise number depending on the genomic and phenotypic complexity of the organisms in a population. Fitness was recorded for each 2 organism and then averaged over all organisms in the population and log10 transformed for statistical analyses. All of the experiments here started from digital organisms that previously evolved in the experiments reported by Misevic et al. (2006). We chose these populations because they are well described and capture a broad range of genetic properties, such as epistasis and modularity, that might affect the evolution of sex. In addition, we chose the Avida system for this study because, although computational in nature, it is much more complex and biological than typical numerical simulations. In essence, it provides an in silico instantiation of open-ended evolution (Wilke and Adami 2002; Lenski et al. 2003; Ofria and Wilke 2004; Adami 2006). Recombination Mechanism After a digital organism has copied its genome (in whole or in part, with or without mutations), the first execution of either divide instruction determines its mode of reproduction and, simultaneously, separates the replicating genome into 2 progeny genomes, as described previously (Misevic et al. 2006). Progeny produced by the divide-sex instruction undergo recombination, whereas those produced by divideasex are immediately placed at random locations in the population. During sexual reproduction, 2 consecutively produced progeny genomes are paired and exchange a single continuous and corresponding region of their circular genomes. These genomes are not necessarily aligned; the identity of recombining regions is based only on the correspondence in their genomic position and not on sequence similarity or homology. The decision to employ this mechanism was motivated by computational efficiency. Recombination based on genomic position probably increases the load of maladapted sexual offspring relative to schemes based on sequence similarity or homology because insertions and deletions disrupt the positional correspondence of related genes. After the exchange of corresponding genomic regions, both offspring are placed at random locations in the population, in the same manner as asexually produced organisms. In our study, organisms do not experience the familiar 2-fold cost of sex because 2 sexual organisms produce 2 offspring. However, digital organisms do experience certain intrinsic costs related to recombination, such as disrupting favorable genetic combinations and combining incompatible genes. Each incipient sexually produced offspring is stored until another such offspring is produced, at which time they recombine their genomes and the 2 recombinant products are placed into the population. By default, the incipient offspring can wait indefinitely, but supplementary experiments in which we limited the maximum waiting time did not qualitatively change evolutionary outcomes (data not shown). Therefore, we decided against introducing another variable in this study. Digital Metabolism An organism’s genome may contain information that encodes the ability to metabolize one or more substrates Downloaded from http://jhered.oxfordjournals.org at Peking University on August 19, 2010 mechanism might provide an advantage to a sexual clade relative to an asexual clade, but whether it can provide an individual-level benefit for sexual reproduction is less clear. Although a single asexual mutant might well be able to invade a sexually reproducing population, 2 sexual organisms are required to gain any foothold in an asexual population (absent the possibility of selfing). We are thus interested in determining whether changing environments can select for the maintenance as well as the initial spread of sexual reproduction. To explore the evolution of sexual reproduction in general, and the role of changing environments in particular, one would like an experimentally tractable evolving system with varying environments and organisms that can mutate between asexual and sexual forms. To that end, we have performed experiments on populations of digital organisms in the Avida system (Wilke and Adami 2002; Lenski et al. 2003; Ofria and Wilke 2004; Adami 2006) to test whether environmental change, including stochastically reversing the direction of selection on some traits, promotes the evolution of sex. Misevic et al. ! Sex In Changing Environments successive generations. The particular pair of substrates that changed their status at any point in time were chosen at random (with a uniform probability distribution) from those in each variable category. Parameters Governing Environmental Change We manipulated the extent of environmental change in 2 respects: by varying the frequency with which the substrates changed from beneficial to harmful or vice versa (Figure 1) and by varying the magnitude of the benefits and costs of metabolizing the substrates. The intervals between changes in the environment were 1, 3, 10, 30, 100, or 300 updates, and the metabolic values between which substrates switched were (–1, 1), (–1, 3), (–1, 5), or (–3, 1), where negative and positive values indicate poisons and nutrients, respectively. For example, when an environmental change occurs during a (–1, 5) treatment, a random nutrient that we can call substrate A is chosen and it becomes a poison, such that its metabolic value changes from 5 to –1. Simultaneously, a random poison that we can call substrate B is chosen and becomes a nutrient, so that its value shifts from –1 to 5. Consider 4 organisms Figure 1. Examples of 3 different periods of change in substrate metabolic values. The 9 fixed substrates and 68 changing substrates are represented by different rows on the y axis. Black cells indicate that a substrate has a positive metabolic value (nutrient), whereas white cells indicate that it has a negative value (poison) at that particular time in an experiment. The light gray region at the left marks the first 1000 updates when all the changing substrates had a metabolic value of zero, allowing the populations to acclimate to their ancestral condition in which only the 9 fixed nutrients were present. Panels (a), (b), and (c) represent environmental change at periods of 3, 30, and 300 updates, respectively. 3 Downloaded from http://jhered.oxfordjournals.org at Peking University on August 19, 2010 present in its environment (Wilke and Adami 2002; Lenski et al. 2003; Ofria and Wilke 2004). Metabolism of a substrate either accelerates or decelerates an organism’s replication rate by a factor of 2m, where m is the substrate’s metabolic value and is positive or negative for a nutrient or poison, respectively. Periodically, one randomly chosen substrate switches from nutrient to poison whereas another substrate simultaneously undergoes the opposite transition. A total of 77 substrates are associated with all of the distinct 1-, 2-, and 3-input logic operations. An organism metabolizes one of these substrates when it executes a sequence of genomic instructions that perform the associated logic operation. Nine substrates (representing the 1- and 2-input operations) that were present during the prior evolution of the ancestors used in our study were again present and always nutritious. The other 68 substrates alternated randomly between being nutrients and poisons, with 25 being nutritious at each moment, according to the treatment schedules described in the next section below. Although some treatment schedules involved changes at periods shorter than generation times, each change affected only 2 of the 68 variable substrates, so that most aspects of the environment were constant across Journal of Heredity that have different genotypes but identical phenotypes, except for the following properties: genotype GØ metabolizes neither substrate A nor substrate B, GA metabolizes only A, GB metabolizes only B, and GAB metabolizes both A and B. Table 1 shows how the fitness of each of these organisms would be affected by this environmental change. For each of the 24 experimental treatments (6 rates of change, 4 magnitudes of change), we propagated 20 initially sexual and 20 initially asexual populations for 100 000 updates, giving a total of 960 populations. Properties of Ancestral Organisms Table 1 Illustration of the effects of an environmental change on the fitness of 4 different genotypes under the (–1, 5) valuation scheme for poisons and nutrients, respectively GØ fitness GA fitness GB fitness GAB fitness Before change W0 After change W0 W0 # 25 W0 # 2$1 W0 # 24 W0 # 2$1 W0 # 25 W0 # 24 The 4 types are phenotypically identical except for their abilities to metabolize substrates A and B. Genotype GØ is unable to metabolize either A or B, and it has fitness W0. By contrast, GA metabolizes only A, GB metabolizes only B, and GAB metabolizes both A and B. Values indicate each genotype’s fitness before (first row) and after (second row) an environmental change whereby substrate A turns from nutrient into poison and, simultaneously, substrate B turns from poison into nutrient. 4 Results and Discussion We begin by examining the rates and magnitudes of environmental change that favor the evolution of sexual reproduction. We then compare populations that started with different modes of reproduction to distinguish between the origin and maintenance of sex. We also analyze the possible influence of genetic architecture on the propensity to evolve sex. Finally, we compare the fitness effects of sexual and asexual reproduction. Effects of Changing Environment on Reproductive Mode The trajectories for the relative abundance of sexual and asexual organisms were highly variable in our experiments, even among replicate populations in the same treatment (Figure 2). Some populations began and mostly remained either sexual (Figure 2h) or asexual (Figure 2c), whereas others switched their mode of reproduction multiple times (e.g., Figure 2f). Sexual and asexual types typically coexisted only for a short period during a transition between reproductive modes, but occasionally both types maintained intermediate frequencies for much longer periods (Figure 2b). Figure 3a summarizes the final numerically dominant mode of reproduction, averaged more than the 40 replicate populations (with half initially sexual and half initially asexual) for each of the 24 treatments. In this analysis, each population was classified as either sexual or asexual based on the most abundant mode of reproduction at the end of the experiment. Asexual reproduction prevailed under most of the conditions tested. However, sexual reproduction tended to be relatively more common at faster rates of environmental change (i.e., as the period of change was shorter), provided also that the benefit of substrates when they were nutrients was large and their cost when poisonous was not. In the most extreme case, when the period of change was 1 update, and the metabolic values of poisons and nutrients were –1 and þ5, respectively, 65% of the populations were predominantly sexual at the end of the experiment. As noted earlier, some populations repeatedly switched their reproductive mode during the evolution experiment (Figure 2). To avoid a potential artifact resulting from the arbitrary choice of the experiment’s duration, we also calculated the proportion of time during which the majority of a population was reproducing sexually. Thus, a population that spent most of its history as asexual would be classified as such, even if it became sexual shortly before the end of the experiment (e.g., Figure 2d). The overall trends are similar, however, whether one considers the final state (Figure 3a) or the proportion of time a population spent Downloaded from http://jhered.oxfordjournals.org at Peking University on August 19, 2010 Our work builds on the experiments of Misevic et al. (2006), in particular information on the genetic architecture of the digital organisms used to seed the populations in this study. Therefore, we must briefly describe our previous results here. The populations in our earlier work evolved either strictly sexually or strictly asexually for more than 10 000 generations. For each evolved population, we measured its average fitness, genome length, modularity, fitness effects of, and interactions between random mutations. We showed that sexual genomes tended to be longer, on average, and had evolved greater modularity in 2 respects. First, those regions of the genome responsible for metabolizing particular nutrients were typically more compact (higher physical modularity, a measure of the mean distance between sites encoding computational traits) in sexual than in asexual organisms. Second, those regions encoding distinct metabolic functions had less overlap (higher functional modularity, a measure of the average overlap in the genomic sites encoding different traits) in sexual than in asexual organisms. Precise definitions of physical and functional modularity can be found in Misevic et al. (2006). To quantify the effects of random mutations as well as their interactions, we constructed millions of single and multiple mutants in each evolved background and measured their fitness values. We fit a power function to those data, log10W 5 –aMb, where W is the average fitness and M is the corresponding number of random mutations. Parameters a and b reflect robustness to individual mutations and the form of mutational interactions (epistasis), respectively. We found that the sexually evolved organisms were, on average, more robust to mutations (asex , aasex) than were the asexual organisms. Interactions among mutations were, on average, antagonistic in both sets, but less so in the sexual than in the asexual organisms (basex , bsex , 1). Misevic et al. ! Sex In Changing Environments dominated by sexually reproducing organisms (Figure 3b). Using the latter data set (in which the dependent variable is continuous rather than discrete), the effects of the period and magnitude of environmental change are both highly significant (2-way analysis of variance [ANOVA]: F5,936 5 17.417, P , 0.001 for the period; F3,936 5 6.135, P , 0.001 for the magnitude), as is their interaction (F15,936 5 10.502, P , 0.001). Origin versus Maintenance of Sex Many authors have reasoned that it is easier to maintain sexual reproduction than for it to evolve de novo given the costs of sexual reproduction (Michod and Levin 1988; Lenski 1999). By starting our experiments with populations of digital organisms that were either entirely sexual or entirely asexual, we can compare the conditions that allow the origin versus maintenance of sex. As noted in the Methods, these digital organisms do not face the widely discussed 2-fold cost of sex because the 2 parental genomes recombine to produce 2 offspring that are both placed in the population. However, selfing cannot occur in this system, and thus sexual reproduction requires 2 parents who, moreover, must be able to produce viable recombinant offspring, perhaps making it difficult for sex to gain a foothold in an asexual population. By contrast, only one asexual mutant is needed to invade a sexual population. Thus, one might expect an asymmetry between the origin and maintenance of sex in this system, even without the 2-fold cost of sex. To examine this issue, we increased the number of replicates to 50 initially sexual and 50 initially asexual populations for the subset of treatments with changing environments where sex was, on average, most successful, specifically with metabolic values switching between –1 and þ5 and with all 6 rates of change used previously, for a total of 600 populations. Across all rates of environmental change, populations were more likely to be predominantly sexual at the end of the experiment if they were initially sexual than if they started as asexual (Figure 4a). Sexual organisms also dominated the populations that started as sexual for a greater proportion of the total time than they did in populations that began as asexual (Figure 4b). The effect of the initial state on the time-averaged dominant mode of reproduction during evolution in changing environments was highly significant (2-way ANOVA, F1,588 5 32.160, P , 0.001), whereas the interaction between the initial mode of reproduction and the rate of environmental change was not significant (F5,588 5 0.850, P 5 0.514). Sex was also more common, regardless of ancestral mode, in more rapidly changing environments (F5,588 5 29.237, P , 0.001). 5 Downloaded from http://jhered.oxfordjournals.org at Peking University on August 19, 2010 Figure 2. Heterogeneity in the proportion of sexual organisms over time in replicate evolving populations. Ancestors were asexual (blue) for panels (a–d) and sexual (red) for panels (e–h). All 8 populations evolved under the same 3-update period and (–1, 5) magnitude of costs and benefits of metabolizing the 68 changing substrates. Journal of Heredity The effect of initial reproductive state might simply indicate a lag time required for the origin and spread of the alternative mode. Alternatively, the evolutionary history prior to the ancestral state might have produced genomic constraints that make it more difficult to switch reproductive mode while retaining overall fitness. To address this issue, we reanalyzed the data by calculating the proportion of time that populations were predominantly sexual during only the second half of the experiments. We observed the same qualitative pattern, with significant effects of the initial mode of reproduction and period of change, but no significant interaction between them (data not shown), though the effect of the initial mode of reproduction was somewhat reduced. Over the entire duration of the experiment, the populations with sexual ancestors were 6 Figure 4. Relationship between the final prevalence of sex and the ancestral mode of reproduction. All populations evolved in the environment with (–1, 5) magnitude of costs and benefits, while the period of environmental change is shown along the x axis. For each period, the final proportion of predominantly sexual populations (panel 4a) and proportion of time spent sexual (panel 4b) were measured in 50 populations that were initially sexual (squares) and 50 others that were initially asexual (diamonds). Error bars show one standard error of the mean. predominantly sexual 38% more often than those with asexual ancestors; in the second half of the experiment, this difference was reduced to 25%. Thus, both factors seem to contribute to the effect of initial state on the evolution of reproductive mode. In any case, sexual reproduction overcame the barriers that hindered its establishment in previously asexual populations about half the time in the most favorable treatments (Figure 4). Effects of Genetic Architecture on the Evolution of Sex Other studies have shown that the reproductive mode influences the evolution of various aspects of genetic architecture including robustness, epistasis, and modularity Downloaded from http://jhered.oxfordjournals.org at Peking University on August 19, 2010 Figure 3. Prevalence of sexual reproduction for populations that evolved in environments with different periods and magnitudes of environmental change. (a) Populations were categorized as sexual if more than half of the organisms at the end of the experiment reproduced sexually. (b) Average proportion of time that evolving populations spent with predominantly sexual reproduction. Error bars represent one standard error of the mean. Misevic et al. ! Sex In Changing Environments Fitness of Sexual and Asexual Populations We also sought to determine whether sexual organisms achieved higher fitness than asexual ones, especially under those conditions where sexual reproduction was most successful. To do so, we compared the average fitness Table 2 Discriminant analyses for the final mode of reproduction under 6 different periods of environmental change Period of change Wilks’s lambda F7,92 P % Correct 1 update 3 updates 10 updates 30 updates 100 updates 300 updates 0.983 0.929 0.902 0.880 0.945 0.981 0.267 1.186 1.694 2.116 0.911 0.298 0.951 0.321 0.131 0.059 0.491 0.937 38 57 56 63 44 47 Each discriminant function was constructed using the ancestor’s fitness, genome length, physical modularity, functional modularity, robustness to individual mutations (a), and coefficient of epistasis (b). P values indicate whether the overall function was significant. Percent correct shows the proportion of 100 evolved populations that were correctly classified as predominantly sexual or asexual at the end of the experiment using the discriminant function. values of sexual and asexual populations that evolved with the (–1, 5) magnitude of change under the 6 different rates of change. We identified the periods of time for each population during which the majority of organisms were either sexual or asexual, and we calculated the mean fitness values during those periods (Figure 5). (Averaging fitness over time is reasonable because, although the environments frequently changed, the numbers of nutrients and poisons did not change.) The mode of reproduction and period of Figure 5. Average fitness of predominantly sexual and predominantly asexual populations. The log10-transformed fitness values while a particular population was predominantly sexual or asexual were separately averaged. The time-averaged fitness levels while sexual (squares) or asexual (diamonds) were then averaged more than the 100 populations that evolved at each period of environmental change. All populations evolved in the environments with (–1, 5) magnitude of costs and benefits of metabolizing the 68 changing substrates. Error bars show one standard error of the mean. 7 Downloaded from http://jhered.oxfordjournals.org at Peking University on August 19, 2010 (Azevedo et al. 2006; Misevic et al. 2006; Lohaus et al. 2010). Here we ask, conversely, whether these features, as reflected in a particular ancestral state, might influence a population’s propensity to evolve sexual reproduction. As shown in the section above, the evolution of sexual reproduction is more difficult than its maintenance. Moreover, organisms may have evolved genetic architectures that are coadapted to their reproductive mode, thus making it more difficult to switch to the alternative mode. For example, favorable gene combinations assembled in asexual lineages might be disrupted by recombination, reducing the fitness of recombinant offspring, thereby impeding the evolution of sex. On the other hand, certain genetic architectures might promote sexual reproduction. First, more modular genomes may allow faster exchange of metabolic building blocks, making sexual organisms more evolvable (Wagner and Altenberg 1996; Earl and Deem 2004; Schlosser and Wagner 2004; Sun and Deem 2009). Second, synergistic epistatic interactions between deleterious mutations may facilitate the removal of those mutations through recombination and selection, thus promoting sexual reproduction (Kondrashov 1982; Michod and Levin 1988; De Visser and Hoekstra 1998; Wolf et al. 2002). To determine whether modularity, epistasis, or other properties of the ancestors (besides reproductive mode itself) influenced the evolution of sex in our experiments, we further analyzed the 600 populations that evolved with the (–1, 5) magnitude of change under the 6 different periods of environmental change. In particular, we ran discriminant analyses separately for each of these 6 treatments, where the objective was to obtain functions that would categorize the final populations as either sexual or asexual based on ancestral fitness, genome length, physical modularity, functional modularity, robustness to individual mutations (a), and strength of epistasis (b). (The measurements of these ancestral properties were performed by Misevic et al. 2006 on 50 sexual and 50 asexual populations that evolved independently for 100 000 updates; see methods and Misevic et al. 2006 for more detailed descriptions of these metrics and previous experiments.) For these analyses, the final population was categorized as sexual or asexual based on the most common reproductive mode at the end of the experiment. The 6 discriminant functions, on average, correctly classified only 50.83% of the populations—hardly better than random guesses—and none was significant (Table 2), even without adjusting significance levels for multiple tests of the same hypothesis. In agreement with recent theoretical work (Misevic et al. 2009), our results therefore indicate that genetic architecture is not strongly predictive of an evolving population’s eventual reproductive mode. Journal of Heredity Conclusions Our experiments show that rapidly changing environments can promote the evolution of sex, at least relative to more slowly changing environments, in this artificial system. At the same time, our results call attention to several limitations of the theory that changing environments will favor the evolution of sexual reproduction: the parameter space that favors sex is quite limited (Figure 3); the origin of sexual reproduction is more difficult than its maintenance (Figure 4); and idiosyncratic effects of ancestry and chance exert strong influences on whether sex evolves (Figure 2). The same or similar limitations are relevant for other theories for the evolution of sex (Otto and Feldman 1997; Otto and Nuismer 2004; Misevic et al. 2004), which has led some researchers to conclude that multiple factors are necessary to account for the evolution of sex (West et al. 1999). In any case, our study shows the utility of digital organisms for testing complex evolutionary theories because they allow one to manipulate relevant features of the environment, control for confounding effects of ancestry, compare the origin and maintenance of organismal traits under the same conditions, and obtain data across many replicate populations for thousands of generations. Of course, any insights gained from experiments with digital organisms may also suggest more focused research on biological systems to examine the generality of those insights. References Adami C. 2006. Digital genetics: unravelling the genetic basis of evolution. Nat Rev Genet. 7:109–118. Azevedo RBR, Lohaus R, Srinivasan S, Dang KK, Burch CL. 2006. Sexual reproduction selects for robustness and negative epistasis in artificial gene networks. Nature. 440:87–90. Barton NH. 1995. A general model for the evolution of recombination. Genet Res. 65:123–144. Bell G. 1982. The masterpiece of nature. Berkeley (CA): University California Press. Charlesworth B. 1976. Recombination modification in a fluctuating environment. Genetics. 83:181–195. Charlesworth B. 1993a. Directional selection and the evolution of sex and recombination. Genet Res. 61:205–224. Charlesworth B. 1993b. The evolution of sex and recombination in a varying environment. J Hered. 84:345–350. Cooper TF. 2007. Recombination speeds adaptation by reducing competition between beneficial mutations in populations of Escherichia coli. PLoS Biol. 5:1899–1905. De Visser J, Hoekstra RF. 1998. Synergistic epistasis between loci affecting fitness: evidence in plants and fungi. Genet Res. 71:39–49. Earl DJ, Deem MW. 2004. Evolvability is a selectable trait. Proc Natl Acad Sci U S A. 101:11531–11536. Gandon S, Otto SP. 2007. The evolution of sex and recombination in response to abiotic or coevolutionary fluctuations in epistasis. Genetics. 175:1835–1853. Goddard MR, Charles H, Godfray J, Burt A. 2005. Sex increases the efficacy of natural selection in experimental yeast populations. Nature. 434:636–640. Hamilton WD. 1980. Sex versus non-sex versus parasite. Oikos. 35:282–290. Kondrashov AS. 1982. Selection against harmful mutations in large sexual and asexual populations. Genet Res. 40:325–332. Kondrashov AS. 1993. Classification of hypotheses on the advantage of amphimixis. J Hered. 84:372–387. Kondrashov AS, Yampolsky LY. 1996. Evolution of amphimixis and recombination under fluctuating selection in one and many traits. Genet Res. 68:165–173. Lenski RE. 1999. A distinction between the origin and maintenance of sex. J Evol Biol. 12:1034–1035. Lenski RE, Ofria C, Pennock RT, Adami C. 2003. The evolutionary origin of complex features. Nature. 423:139–144. Lively CM. 1987. Evidence from a New Zealand snail for the maintenance of sex by parasitism. Nature. 328:519–521. Lively CM, Dybdahl MF. 2000. Parasites adaptation to locally common host genotypes. Nature. 405:679–681. Funding US National Science Foundation (CCF-0643952) to C.O.; DARPA ‘Fun Bio’ Program (HR0011-05-1-0057) to R.E.L. and C.O. Acknowledgements We thank Ricardo Azevedo and 2 anonymous reviewers for their helpful comments on an earlier version of our paper. D.M. also thanks John Logsdon and Maurine Neiman for organizing the symposium at which this work was presented. 8 Lohaus R, Burch CL, Azevedo RBR. 2010. Genetic architecture and the evolution of sex. J Hered. Malmberg RL. 1977. Evolution of epistasis and advantage of recombination in populations of bacteriophage T4. Genetics. 86:607–621. Maynard Smith J. 1978. The evolution of sex. Cambridge (UK): Cambridge University Press. McPhee CP, Robertson A. 1970. The effect of suppressing crossing-over on the response to selection in Drosophila melanogaster. Genet Res. 16:1–16. Michod RE, Levin B, editors. 1988. The evolution of sex. Sunderland (MA: Sinauer. Misevic D, Kouyos RD, Bonhoeffer S. 2009. Predicting evolution of sex on complex fitness landscapes. PLoS Comp Biol. 5:e1000510. Downloaded from http://jhered.oxfordjournals.org at Peking University on August 19, 2010 environmental change had significant effects on fitness, as did their interaction (2-way ANOVA: F1,1188 5 8.930, P 5 0.003 for reproductive mode; F5,1188 5 8.068, P , 0.001 for period of change; F5,1188 5 2.882, P 5 0.014 for interaction). In the rapidly changing environments, those populations dominated by sexual organisms typically had higher average fitness than did predominantly asexual populations (Figure 5). By contrast, in the slowly changing environments, although most populations evolved to be predominantly asexual (Figure 3), the individuals in sexual and asexual populations achieved similar average fitness levels (Figure 5). This pattern calls attention to potential discrepancies between individual and population metrics of success when it comes to reproductive mode, an issue that merits further study. Misevic et al. ! Sex In Changing Environments Misevic D, Ofria C, Lenski RE. 2004. Sexual reproduction and Muller’s ratchet in digital organisms. In Proceedings of Artificial Life IX. In: Pollack JB, Bedau M, Husbands P, Ikegami T, Watson RA, editors. Cambridge (MA): MIT Press. pp. 340–345. Schlosser G, Wagner GP, editors. 2004. Modularity in development and evolution. Chicago (IL): University Chicago Press. Misevic D, Ofria C, Lenski RE. 2006. Sexual reproduction reshapes the genetic architecture of digital organisms. Proc R Soc Lond B Biol Sci. 273:457–464. Van Valen L. 1973. A new evolutionary law. Evol Theor. 1:1–30. Ofria C, Wilke CO. 2004. Avida: a software platform for research in computational evolutionary biology. Artif Life. 10:191–229. Otto SP, Felman MW. 1997. Deleterious mutations, variable epistatic interactions, and the evolution of recombination. Theor Popul Biol. 51:134–147. Otto SP, Nuismer SL. 2004. Species interactions and the evolution of sex. Science. 304:1018–1020. Peters AD, Lively CM. 1999. The Red Queen and fluctuating epistasis: a population genetic analysis of antagonistic coevolution. Am Nat. 154:392–405. Rice WR. 2002. Experimental tests of the adaptive significance of sexual recombination. Nat Rev Genet. 3:241–251. Wagner GP, Altenberg L. 1996. Complex adaptations and the evolution of evolvability. Evolution. 50:967–976. Waxman D, Peck JR. 1999. Sex and adaptation in a changing environment. Genetics. 153:1041–1053. Weismann A. 1889. Essays upon heredity and kindred biological problems. Oxford: Clarendon Press. West SA, Lively CM, Read AF. 1999. A pluralist approach to sex and recombination. J Evol Biol. 12:1003–1012. Wilke CO, Adami C. 2002. The biology of digital organisms. Trends Ecol Evol. 17:528–532. Wilke CO, Wang JL, Ofria C, Lenski RE, Adami C. 2001. Evolution of digital organisms at high mutation rates leads to survival of the flattest. Nature. 412:331–333. Wolf JB, Brodie ED, Wade MJ, editors. 2002. Epistasis and the evolutionary process. Oxford: Oxford University Press. Salathe M, Kouyos RD, Bonhoeffer S. 2008. The state of affairs in the kingdom of the Red Queen. Trends Ecol Evol. 23:439–445. Received October 8, 2009; Revised January 14, 2010; Accepted January 27, 2010 Sasaki A, Iwasa Y. 1987. Optimal recombination rate in fluctuating environments. Genetics. 115:377–388. Corresponding Editor: John Logsdon 9 Downloaded from http://jhered.oxfordjournals.org at Peking University on August 19, 2010 Otto SP, Michalakis Y. 1998. The evolution of recombination in changing environments. Trends Ecol Evol. 13:145–151. Sun J, Deem MW. 2009. Spontaneous emergence of modularity in a model of evolving individuals. Phys Rev. E 79:031907.