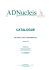Kinetic amplification of enzyme discrimination.
Transcription
Kinetic amplification of enzyme discrimination.
BIOCHIMIE, 1975, 57, 587-595.
Kinetic amplification of enzyme discrimination.
J a c q u e s NINIO .,@ .
The Salk Institute [or Biological Studies P.O. Box 1809,
San Diego, Ca!i[ornia 92112.
(12-12-197/t).
Summary - - The dependance of the accuracy of enzymatic systems on the mechanism
of the catalyzed reaction is investigated, using a prohabilistic approach. Certain mechanisms of reaction, involving a delay in one of the steps act as kinetic amplifiers of
molecular discriminations. The relationship between our scheme for a delayed reaction [1] and Hopfield's scheme [2] is discussed.
INTRODUCTION.
Many enzyme systems discriminate with remark a b l e e f f i c i e n c y b e t w e e n t h e i r s u b s t r a t e a n d clos e l y r e l a t e d m o l e c u l e s . It is g e n e r a l l y a c c e p t e d
t h a t t h i s d i s c r i m i n a t i o n d e p e n d s in l a r g e m e a s u r e
on an a c c u r a t e m a t c h i n g of the s h a p e of t h e
e n z y m e to that of t h e s u b s t r a t e e i t h e r d u r i n g
b i n d i n g of the l i g a n d o r at a s u b s e q u e n t step of
t h e r e a c t i o n ~3, 4].
T h e e x a m i n a t i o n of r e c e n t r e s u l t s (to be disc u s s e d later) on the a c c u r a c y of D N A p o l y m e r i zation, on r i b o s o m a l s e l e c t i o n a n d on the d i s c r i m i n a t i v e a b i l i t i e s of the a m i n o a e y l - t R N A ligases
led us to m o d i f y t h a t p o i n t of v i e w , at least for
c e r t a i n classes of e n z y m a t i c s y s t e m s . Our p i c t u r e
involves two elements :
a) An e n z y m e h a s c e r t a i n d i s c r i m i n a t i v e abilities b y v i r t u e of its m a t c h i n g to t h e s u b s t r a t e .
T h i s m a y be r e f l e c t e d t h r o u g h a d i f f e r e n c e in the
d i s s o c i a t i o n c o n s t a n t s k s a n d k~ of t h e e n z y m e s u b s t r a t e a n d the e n z y m e - a n a l o g u e a s s o c i a t i o n s ,
or t h r o u g h d i f f e r e n c e s in o t h e r k i n e t i c p a r a m e t e r s of the r e a c t i o n .
b) T h e e n z y m a t i c r e a c t i o n is c o n s i d e r e d as a
p r o c e s s i n g of t h e s u b s t r a t e a n d t h e a n a l o g u e
l e a d i n g to p r o d u c t s p s a n d pA. T h e p r o c e s s as a
w h o l e c o n t a i n s o n e (or m o r e ) d i s c r i m i n a t i v e
step, a n d t a k e s m o r e o r less a d a v a n t a g e of the
discriminative
potentialities
r e f l e c t e d in t h e
ratio k 2/k s
O u r e x a m i n a t i o n of s e v e r a l cases s h o w in
a g r e e m e n t w i t h i n t u i t i o n , t h a t in m o s t cases, the
d i s c r i m i n a t i o n of t h e w h o l e p r o c e s s is s m a l l e r
Present address where all correspondence shouht
be addressed: Institut de Biologic Mol6culaire, Tour 43,
2 Place Jussieu, Paris (58).
t h a n t h a t of t h e d i s c r i m i n a t i v e step. T h u s , if t h e
c o n c e n t r a t i o n s of s u b s t r a t e a n d a n a l o g u e a r e
equal, w e h a v e in g e n e r a l [ p s ] / E p A ] ~-- kaA / k as.
H o w e v e r , w e l o o k e d for a n d f o u n d a class of
r e a c t i o n s w h i c h a l l o w the p r o c e s s as a w h o l e to
be m o r e d i s c r i m i n a t i v e t h a n its d i s c r i m i n a t i v e
step. W e are a b l e to c o n s t r u c t s c h e m e s f o r
w h i c h [ p s ] / [ p A ] m a y a p p r o a c h (]~c~/ks )~ o r e v e n
h i g h e r p o w e r s of t h e r a t i o of t h e t w o d i s s o c i a t i o n
c o n s t a n t s (*).
T h u s , in p r i n c i p l e , N a t u r e has at least t w o
s t r a t e g i e s for a c h i e v i n g a c c u r a c y . It m a y d e s i g n
a v e r y s o p h i s t i c a t e d b i n d i n g site, o r it m a y s e l e c t
a s h r e w d m e c h a n i s m of r e a c t i o n w h i c h t a k e s
a d v a n t a g e of s m a l l d i f f e r e n c e s in k i n e t i c p a r a meters. The second strategy would appear more
i n t e r e s t i n g in s i t u a t i o n s w h e r e t h e e n z y m e s y s t e m
n e e d s to s o l v e several a c c u r a c y p r o b l e m s . T h i s is
p r e c i s e l y the case e n c o u n t e r e d in D N A p o l y m e r i z a t i o n a n d in r i b o s o m a l s e l e c t i o n of tRNA.
DNA polymerization. A s a m e e n z y m e is i n v o l v e d in the r e p l i c a t i o n of e a c h of t h e f o u r bases
A, T, G, C. d C T P w h i c h is a s u b s t r a t e in one case
( w h e n a G is c o p i e d ) b e c o m e s a d a n g e r o u s
a n a l o g u e w i t h r e s p e c t to t h e t h r e e o t h e r cases.
M u t a n t s of p h a g e T4 D N A p o l y m e r a s e h a v e
b e e n o b t a i n e d , d i s p l a y i n g e i t h e r m n t a t o r [5] or
a n t i m u t a t o r [6] p r o p e r t i e s . T h e s e m u t a n t s h a v e
been extensively characterized both genetically
[7-91 a n d b i o c h e m i c a l l y [10-13]. T h e i n t e r e s t i n g
(*) Our findings were briefly presented at <<Eeole
d'Et6 sur l'Evolution des Macromol6cules Biologiques >>
(Roscoff, May 1974) and are discussed in the proceedings of that meeting [1]. After the submission of
this more com~plcte paper, John Hopfield's article
bearing on the same subject [2] reached us, and the
manuscript was revised in order to include a comparison between the two approaches and discuss recent
experimental findings.
J. N i n i o .
588
feature is that different p o i n t m u t a t i o n s in the
DNA p o l y m e r a s e gene have the effect of l o w e r i n g
or r a i s i n g s i m u l t a n e o u s l y most of the error-levels
that can be m e a s u r e d : s p o n t a n e o u s A.T.
~
G.C or G . C - - - - ~ - A . T t r a n s i t i o n s , t r a n s v e r s i o n s ,
errors due to the i n c o r p o r a t i o n of base-analogues
such as 2-amino p u r i n e or bromo-U, etc. [9]. Yet,
the a m i n o a c i d substitutions resulting from the
m u t a t i o n s should have different geometrical consequences.
This suggests that T4 DNA p o l y m e r a s e makes
use of a d i s c r i m i n a t i v e p r o c e d u r e w h i c h can be
a p p l i e d w i t h more or less vigour. More precisely,
it was proposed that the l d n e t i c b a l a n c e between
two steps of the reaction ( p o l y m e r i z i n g activity
versus exonuclease activity) was more i m p o r t a n t
for DNA p o l y m e r a s e selectivity than the discrim i n a t i v e potentialities of the i n d i v i d u a l steps
[13]. U n f o r t u n a t e l y , the authors reached that conclusion on the basis of a kinetic t r e a t m e n t w h i c h
is actually i r r e l e v a n t (see the Discussion).
R i b o s o m a l selection. Here again, a n u m b e r of
r i b o s o m a l m u t a n t s are k n o w n w h i c h have the
effect of c h a n g i n g i n a parallel m a n e r <<n a t u r a l
errors>>, and all the levels of n o n s e n s e a n d
missense s u p p r e s s i o n [14:1~;]. Judged from the
p o i n t of view of the geometry of the codon-anticodon i n t e r a c t i o n s , the various effects appeared
c o n t r a d i c t o r y [17~. However, they could be reduced to a single effect w h e n geometry considerations w e r e a b a n d o n n e d . T h e y could be a c c o u n t e d
for by a change i n the d i s c r i m i n a t i v e abilities of
the ribosome [18] e x p l a i n e d by the change of a
single k i n e t i c p a r a m e t e r in the r e a c t i o n [1'9].
UNDELAYED REACTIONS.
We start our study of the d i s c r i m i n a t i v e
<<s h r e w d n e s s >) of v a r i o u s r e a c t i o n schemes w i t h
the classical Michaelis model :
ld
ke,
E + S ~ "
ES . . . .
~,-E 4. ps
(1)
We assume t h r o u g h o u t the article that such
m a c r o s c o p i c r e p r e s e n t a t i o n s are exact, i.e., they
reflect a c c u r a t e l y the processes at the m i c r o s c o p i c
level. T h e n the k i n e t i c s can be expressed in terms
of probabilities. IS? k s dt is the p r o b a b i l i t y dp
that a free enzyme molecule w i l l form an enzymesubstrate complex in the i n f i n i t e s i m a l time dr,
w i t h s i m i l a r expression for k s a n d k_s. W h e n the
complex ES is formed, it disappears exponentially w i t h time a c c o r d i n g to exp (-kS t &st).
k S is the r e c i p r o c a l of the mean s t i c k i n g time
BIOCHIMIE, 1975, 57, n ° 5.
~s of the complex, that w o u l d be measured i n the
absence of the p r o d u c t - f o r m i n g reaction.
Once an enzyme-substrate or an enzyme-analogue association is formed, h o w e v e r t r a n s i t o r y it
may be, what is the p r o b a b i l i t y of c o m p l e t i o n of
the reactio.n ? F o r a n y short i n t e r v a l of time dt,
a fraction k~ decays, w h i l e a fraction k s dt leads
to p r o d u c t formation. Thus, the average probability of c o m p l e t i o n of the reaction is quite
s i m p l y given by P = k s / ( k s -]- kS1) and by the
e q u i v a l e n t expression for the analogue A. If we
c o m p a r e the rates at w h i c h substrate and analogue are t r a n s f o r m e d , we have the ratio :
Vs
ESI k s ps
- -
~ -
VA
(2)
[A] ki~ pA
We will define the d i s c r i m i n a t i o n term D as
the velocity ratio of Eq (2) divided by the conc e n t r a t i o n ratio :
Vs / VA
1)
(3)
E s I . EAI
In the case of Michaelis kinetics, D is a product of two terms : the ratio of the collision efficien~cies of substrate and analogue, a n d the ratio
of the p r o b a b i l i t i e s of completion of the reaction
after a collision :
k s pS
D -(4)
k~ pa
This expression can be extended to a n y other
m e c h a n i s m of r e a c t i o n for w h i c h there is only
one c o n f o r m a t i o n a l state of the enzyme a l l o w i n g
an e n t r y of the ligands.
Consider the special ease w h e r e k s = k la
a n d k s = k 2 = A k2 " W h e n the reaction time I/ko
is very short c o m p a r e d to both s t i c k i n g times
I/kSl and I / k ~ , D = I. The enzyme does not
d i s c r i m i n a t e b e t w e e n substrate and analogue. As
the r e a c t i o n time increases w i t h respect to the
s t i c k i n g times, d i s c r i m i n a t i o n also increases and
reaches the l i m i t D = k~/1Csl = {-sfiA for infinite
r e a c t i o n times. Thus, w h e n the only kinetic difference w h i c h is exploited by the enzyme is that
of the sticking times, d i s c r i m i n a t i o n is at best i n
the ratio of the stictdng times. (Of course, a
larger D value can be o b t a i n e r if kS2 > k2)'a
The result can be generalized to the case w h e r e
there are a d d i t i o n a l i n t e r m e d i a t e stages in the
f o r m a t i o n of the p r o d u c t :
k~
kn + I
. . . . . ~ : : _ " ~ ' E S ( ~ , ) - - - ~ E ÷ ps
(5)
Enzgme
W h e n tile c o m p l e x ES(~) is f o r m e d , it m a y
either dissociate, or yield product with a probab i l i t y P w h i c h is g i v e n b y the e l e g a n t f o r m u l a
of K n o r r e a n d M a l y g u i n e [20~.
I
P
k 1 (
--I+
[
I +
~-2
....
k
i I+
-"
ka
+ k-(n-j~) (t + - - - - )
kn
n]i
589
Discrimination.
(6)
of c o n v e n t i o n a l c h e m i c a l events. T h e n , t o is n o t
a fixed time, but a random variable with a mean
v a l u e t h a t c a n be c o m p u t e d .
T h e f o l l o w i n g is t h e s i m p l e s t e x a m p l e of
d e l a y e d r e a c t i o n s w e h a v e b e e n a b l e to c o n s t r u c t .
W e s t a r t w i t h a t w o - s u b s t r a t e r e a c t i o n in w h i c h
t h e b i n d i n g of o n e s u h s t r a t e h a s no i n f l u e n c e on
t h e b i n d i n g of the o t h e r s u b s t r a t e (see Eq. (7)).
kn+l
F r o m t h e r e , it is easy to s h o w that w h e n all
the k i n e t i c p a r a m e t e r s of s u b s t r a t e a n d a n a l o g u e
s a v e o n e p a i r (k s a n d k ; ' or kS a n d k A) are equal,
the d i s c r i m i n a t i o n c a n n o t be b e t t e r t h a n t h e r a t i o
of t h e k i n e t i c p a r a m e t e r s : k ~ / k s o r k Ai / k . iS. T h e
r e s u l t a p p l i e s to a n y p a i r of k i n e t i c p a r a m e t e r s ,
a n d to a r e a c t i o n i n v o l v i n g a n y n u m b e r of steps.
DELAYED REACTIONS.
In this section we consider the case where
b i n d i n g is the d i s c r i m i n a t i v e step. W e c o u l d h a v e
said, for a M i c h a e l i s s c h e m e , t h a t t h e e n z y m e is
c eomparing>> t h e s t i c k i n g t i m e s ~s and Ta.
S u p p o s e n o w t h a t t h e r e a c t i o n s u b s e q u e n t to
b i n d i n g of the s u b s t r a t e or t h e a n a l o g u e is
d e l a y e d b y a t i m e t o. T h e t i m e s d u r i n g w h i c h
the r e a c t i o n s m a y o c c u r are n o w ~'s = ~ s __ to
and ( a
= ( a __ to . It is c l e a r t h a t t h e r a t i o
t ' s / t ' a of t h e s e a p p a r e n t s t i c k i n g t i m e s can be
c o n s i d e r a b l y l a r g e r t h a n the r a t i o t s / t a. T h i s
r a i s e s t h e q u e s t i o n : a r e snell d e l a y e d r e a c t i o n s
c o n c e i v a b l e c h e m i c a l l y ? At first, o n e m a y t h i n k
that t h e r e a l i z a t i o n of d e l a y s s h o u l d i n v o l v e
effects w h i c h a r e o u t s i d e t h e r a n g e of v a l i d i t y
of tile t r a d i t i o n a l c h e m i c a l n o t a t i o n . It t u r n s out
h o w e v e r t h a t d e l a y s c a n be o b t a i n e d b y s e q u e n c e s
and we make the E ~
E S 2 b r a n c h of the
r e a c t i o n i r r e v e r s i b l e b y c o u p l i n g it to a v e r y
rapid destructive process, for instance :
ES.)_ + A T P
(very rapid)
>
E -t- $2 + AMP + PP
(8)
or, if S 2 is A T P , this last r e a c t i o n can be r e p l a c e d
b y the in situ d e g r a d a t i o n of A T P .
T h e r e a s o n f o r the a d d i t i o n a l (and
r e a c t i o n w i l l be m a d e i n t u i t i v e later.
essential)
Let us i n t r o d u c e t h e v a r i o u s p r o b a b i l i t i e s of
the d i r e c t t r a n s i t i o n s f r o m one state to a n o t h e r :
p : k 2 !S,,!/(k 2 [$2] + k l ) f o r E S i
q = k a / ( k a + k 2 + k l) for ES1S 2
r -- k e/(k a + k_2 + k l) for ES1S 2
-~- ESaS 2
_~P, and
~ ES 1
A g a i n w e ask the q u e s t i o n : o n c e ES t is f o r m e d ,
w h a t is the p r o b a b i l i t y t h a t it w i l l l e a d to P ? W e
may have several back and forth motions froul
ES1S.~ to ES 1 a n d ES~ to ES1S 2 b e f o r e f o r m i n g the
p r o d u c t . T h u s , the o v e r a l l p r o b a b i l i t y is g i v e n as
the s u m of the s e r i e s :
P
=
1)q + p r p q
+
(pr)2pq
+
......
(9)
Pq
1 --
pr
k.~ k s [$21
(lO)
- - k , 2 k a [$2] + k_1 (k 3 + k_2 -}- k 2 IS2]) + k-'21
ES1
ka
ES z
BIOCHIMIE, 1975, 57, n ° 5.
5
E + 1'
(7)
J. Ninio.
590
T h e i n t e r e s t i n g f e a t u r e is t h e p r e s e n c e of a
q u a d r a t i c k_21 t e r m . Fo.r v e r y s h o r t s t i c k i n g t i m e s
( l a r g e v a l u e s of k 1), a n d if t h e o n l y k i n e t i c differ e n c e w h i c h is e x p l o i t e d is t h a t of s t i c k i n g t i m e s ,
we have :
I) ~
(ts/ta)2
p r o b a b i l i t y of p r e s e n c e is zero. Sz m a y c o m e ,
leave, c o m e again, etc. Its p r o b a b i l i t y of p r e s e n c e
r i s e s g r a d u a l l y u n t i l it r e a c h e s a m a x i m u m l e v e l
(11)
Thus, analogues
with very weak binding
c o n s t a n t s a r e p r o c e s s e d a c c o r d i n g to t h e s q u a r e s
of t h e s t i c k i n g t i m e s .
In o r d e r to d e t e r m i n e m o r e p r e c i s e l y the
d o m a i n of v a l i d i t y of t h e q u a d r a t i c effect, o n e
has to t a k e i n t o a c c o u n t t h e t w o b r a n c h e s of t h e
r e a c t i o n . L e t us c a l l a t h e c o n c e n t r a t i o n of E r e l a tivetoES~:
a = [ E ] / ( [ E ] -4- [ E S 2 J ) - - u c a n b e
c o m p u t e d t h r o u g h t h e s t e a d y - - state e q u a t i o n s .
T h e c o m p l e t e e x p r e s s i o n for P i s :
P
Pq
= a -I-pr
q
-4- ( I - a ) - - I-pr
(12)
T h e q u a d r a t i c e f f e c t is f o u n d in t h e p q / ( I - p r )
t e r m , a n d n o t in t h e q / ( I - p r ) t e r m . T h e i r r e v e r sible c o u p l i n g of E q u a t i o n (8) h a s t h e effect of
n m k i n g a close to o n e a n d t h u s is a n e c e s s a r y
c o n d i t i o n for t h e v a l i d i t y of E q u a t i o n (10). T h e
q u a d r a t i c effect r e q u i r e s a l a r g e v a l u e of k. 1
( s u p e r i o r to k 3, k 2 a n d k 2 [$2]) b u t n o t too l a r g e
a v a l u e e i t h e r , f o r t h e n t h e (I-~) q / ( I - p r ) t e r m
w o u l d c e a s e to be n e g l i g i b l e c o m p a r e d to t h e
u p q / ( I - p r ) t e r m . T h u s , t h e q u a l i t y of t h e i r r e v e r s i b l e c o u p l i n g ( r e f l e c t e d in t h e v a l u e of a) d e t e r m i n e s h o w f a r in t h e d o m a i n of s h o r t s t i c k i n g
times, the a m p l i f i c a t i o n effect m a y h o l d .
T h e q u a d r a t i c effect is a b s e n t w h e n t h e t w o stcp r e a c t i o n f o l l o w s a m e c h a n i s m of s h ' i c t l y
o r d e r e d a d d i t i o n s u c h as :
kl
E -4- $ 1 ~
ESj
I~k2
ES1 -4- S,~~.-~>ES1S2
k_2
k3
~-E -4- P
(13)
T h i s r e a c t i o n c a n be r e w r i t t e n as :
kl
E+
1
ESI
k'2 [S2] >ES1S,,.
K
k3
~ E + P
timo
Fro. 1. - - Intuitive explanation of the kinetic amplification effect.
We consider a two-substrates reaction (Equations
(7) and (8)). The first substrate binds to the enzyme
with a sticking time 0 s larger than the sticking time
0A of the analogue A. ~Ve take as origin of the timeaxis the instant w h e n the complex ES~ or EA is forreed. The probability of presence of $2 on the enzyme
(P, in ordinate) increases 'with time. The asymptotic
v~tlue of P corresponding to equilibration between
arrivals and departures of S~ is k2 [S~]/(k~ [S~l + k_2).
The overall probability of the reaction w h e n $1 or A is
on the enzyme can be obtained by adding the probabilities of the reaction during consecutive intervals of
time. For every small interval of time St, the probability of the reaction is the product of the probability
of presence of $2 (the value of P taken on the curve)
by a constant ~dt. ~ht is the probability of forming
a product during a small interval of time 8t vchen
both S~ and S~ are on the enzyme. Thus, the overall
probabilities corresponding to sticking time 0 s and 0A
are proportional to the areas under the curve. For
small O's they vary as the squares of sticking times.
c o r r e s p o n d i n g to e q u i l i b r a t i o n (fig. 1). S u p p o s e
w e d i v i d e t h e t i m e - a x i s in s h o r t s u c c e s s i v e i n t e r vals o,f d u r a t i o n St. T h e s t i c k i n g t i m e s of snbs t r a t e a n d a n a l o g u e can be w r i t t e n as NS fit a n d
NA 8t r e s p e c t i v e l y , w h e r e N s a n d N A c a n b e t a k e n
as i n t e g e r s .
D u r i n g an i n t e r v a l of t i m e St, a n d p r o v i d e d
b o t h S~ a n d S 1 a r e p r e s e n t on t h e e n z y m e , t h e r e
is a p r o b a b i l i t y 8p of m a k i n g t h e p r o d u c t .
I n i t i a l l y , t h e p r o b a b i l i t y of p r e s e n c e of S 2 is l o w
and what happens makes only a small contribut i o n to t h e total p r o b a b i l i t y of f o r m i n g t h e
product.
_
(14)
and the discrimination
E q u a t i o n (6).
can
be
obtained
from
T h e r a t i o of t h e t w o p r o b a b i l i t i e s f o r s u b s t r a t e
a n d a n a l o g u e (if t h e i r s t i c k i n g t i m e s a r e s h o r t
e n o u g h ) c a n be a p p r o x i m a t e d b y (see F i g . 1) :
ps/pA
Intuitive explanation of the delayed reaclion
effect. Let us c o n s i d e r t h a t t h e c o m p l e x E S 1 h a s
b e e n f o r m e d at t i m e 0, a n d let us f o l l o ~ t h e
e v o l u t i o n of t h e e n , z y m e - s u b s t r a t e c o m p l e x w i t h
time. I n i t i a l l y , S 2 is absent f r o m t h e c o m p l e x : its
BIOCHIMIE, 1975, 57, n ° 5.
= (1.4.2.4.3.4. . . . . -4- N s) 8 P /
(1.4.2.4.3.4. . . . . -4- NA)SP
(NS,/NA) 2
(15)
(This was indeed the intuitive consideration
w h i c h led us to the c o n s t r u c t i o n of t h e r e a c t i o n
scheme).
Enzyme Discrimination.
The p r e c e d i n g p r o b a b i l i s t i c d e s c r i p t i o n does
not a p p l y to strictly ordered reactions. F o r then,
u p o n b i n d i n g of So, S1 is not allowed to leave the
complex p r i o r to the d e p a r t u r e of S,), a n d the
s t i c k i n g time of S 1 c a n n o t he defined as an entity
i n d e p e n d a n t of the b i n d i n g of S~.
Thus, the p r o b a b i l i s t i c model i m p l i e d r a n d o m
d e p a r t u r e s for S1 a n d S.,. If this c o n d i t i o n is
realized, the p r i n c i p l e of m i c r o s c o p i c reversibility requires that the order of b i n d i n g of S1 and
S2 be also r a n d o m , a n d we are led to scheme (7).
However, we are interested in the u p p e r b r a n c h
of the reaction, exclusively. The lower b r a n c h
can d i s c r i m i n a t e at best in the ratio of sticking
times. F r o m there, the necessity of E q u a t i o n (8)
follows n a t u r a l l y .
COMPARISON W I T H HOPFIELD'S SCHEME.
591
In both schemes, there are two gates of exit for
the substrate, a l l o w i n g a double-cheek mechanism. Since the substrate could bypass the doublecheck if e n t e r i n g directly through the second
gate, an i r r e v e r s i b l e energetic c o u p l i n g is introduced in both schemes. It has the effect of m a k i n g
the second gate p r a c t i c a l l y inaccessible for substrate entry. The m a i n difference b e t w e e n the
two schemes c o n c e r n s the p o s i t i o n n i n g of the
i r r e v e r s i b i l i t y w i t h respect to the last step of the
reaction.
Physically, while our scheme c o r r e s p o n d s to
an a u t h e n t i c time-delay, Hopfield's scheme corresp o n d s to a p u m p i n g effect analogous to the Overhauser effect in N.M.R., a n d was discovered
t h r o u g h this analogy (Hopfield, p e r s o n a l communication).
DELAYED ESCAPE OF THE PRODUCT.
In the p r e c e d i n g example b i n d i n g was the
d i s c r i m i n a t i v e step. We p r e s e n t n o w a scheme
Hopfield's scheme can be w r i t t e n a s :
ES
(t~i)
E
AMP + P P i
ES"
>
Let us intrc~duce the e l e m e n t a r y p r o b a b i l i t i e s
of t r a n s i t i o n :
p = ke./(k,~ -[- k l) for ES
-~'ES*
q -- k3/(k 3 + k_,2 + k. 4) for ES*
---~" P
r : k_z/(k 3 q-- k_,~ + k_~) for ES*
--~- ES
W h e n the substrate b i n d s to E, it m a y go either
to ES w i t h the f r e q u e n c y a : k l / ( k 1 q- k 4) or to
ES* w i t h the f r e q u e n c y I-a. Thus, the overall
p r o b a b i l i t y of f o r m i n g the p r o d u c t after a collision b e t w e e n the enzyme a n d the substrate is :
P :
Pq
~-I-pr
q
+ (I-a)-I-pr
(17)
Kinetic amplification is o b t a i n e d as i n our
example through the p q / ( I - p r ) term. T h e r e is a
complete m a t h e m a t i c a l analogy b e t w e e n the two
schemes.
BIOCHIMIE, 1975, 57, n ° 5.
E+P
w h e r e i n the amplification effect is exerted u p o n
a step of a different nature. The model is directly
i n s p i r e d by k n o w n biological facts.
Some DNA polymerases Ell 12, 21, Z2] and some
aminoaeyl-tRNA ligases [23, 24] are k n o w n to
possess a h y d r o l y t i c activity t o w a r d s the p r o d u c t
of their reaction, w h i c h is i n d e p e n d a n t of their
synthetic activity (the latter b e i n g d r i v e n by the
splitting of a p y r o p h o s p h a t e bond). Consider an
isoleucyl-tRNA ligase w h i c h has just aminoacylated a molecule of tRNAne w i t h either isoleucine or valine. While the aeylated tRNA is still
on the enzyme it is subjected to a h y d r o l y t i c
activity with rates kS and k A for Ile-tRNAne a n d
val-tRNAI~e respectively. Let us call t the time
that the loaded tRNA s p e n d s on the enzyme. The
p r o b a b i l i t i e s of h y d r o l y s i s for the two molecules
are I-exp(-kSt) and I-exp(-kAt) respectively. It is
J. Ninio.
592
easy to show from there that the ratio of h y d r o lyzed Val-tRNAn~ to hydro!yzed Ile-tRNAIlo~
c a n n o t exceed kA/kS. Howver, this ka/kS ratio
is not the relevant one. What is really i m p o r t a n t
is the ratio of the surviving molecules of the two
kinds. Then, the a n s w e r is quite different. If the
ratio of correct to i n c o r r e c t molecules b o u n d to
the enzyme before the h y d r o l y t i c step is pS/pA,
it becomes after that step :
ps/pa
= pS/p.& e-(kS-kA)t
(18)
This suggests the possibility that an enzyme
m a y achieve an e x p o n e n t i a l exploitation of differences in k i n e t i c parameters. Here, we r e a s o n n e d
as if the time t available for the h y d r o l y s i s of the
p r o d u c t was a fixed time. This c o n d i t i o n is not
realized i n the most simple situatic, n, w h e n ~he
p r o d u c t leaves the enzyme a c c o r d i n g to firstorder kinetics with a r a t e - c o n s t a n t k. We w o u l d
obtain :
ps/pA
= pS/pA. (k ÷ k a ) / ( k + kS)
(19)
a n d the c o n t r i b u t i o n of the p r o o f - r e a d i n g function to d i s c r i m i n a t i o n w o u l d not be better t h a n
the ratio kA/kS.
However, if the escape of the p r o d u c t is
delayed, although t r e m a i n s a r a n d o m variable,
its d i s t r i b u t i o n is more stepwise a n d one o b l a i n s
an amplification effect. As a practical w a y of
a c h i e v i n g a (random) delay, c o n s i d e r the foliow i n g model. Upon a m i u o a c y l a t i o n , the tRNA is
firmly hooked to the enzyme. Its d e p a r t u r e
r e q u i r e s a e o n f o r m a t i o n a l change of the IRNA, or
of the enzyme, or the b i n d i n g of a n o t h e r molecule
to the enzyme, for i n s t a n c e the b i n d i n g of a
second a m i n o acid [~6, 27~. The overall situation
can be described b y the scheme :
the p r e c e d i n g section. W h e n ES~ is formed, the
p r o b a b i l i t y of escaping h y d r o l y s i s is o b t a i n e d
r e p l a c i n g i n E q u a t i o n (10) k 1 by kS. Now, the
amplification effect bears on the h y d r o l y s i s rates,
and not on sticking times.
THE RANDOM WALK OF A DNA POLYMERASE.
The situation w i t h the b i f u n c t i o n a l DNA polymerases presents a d d i t i o n a l i n t e r e s t i n g features.
After the template-directed i n c o r p o r a t i o n of a
b.ase (n) the enzyme moves one step f o r w a r d for
the i n c o r p o r a t i o n of the next base (n-+-1).
Meanwhile, the base i n c o r p o r a t e d last is (<tested >>
by the exonucleolytic f u n c t i o n . If h y d r o l y s i s
occurs, the enzyme moves one step b a c k w a r d s .
On the other h a n d , the i n c o r p o r a t i o n of the base
(n ÷ 1) by the head of the enzyme, w i t h the
s u b s e q u e n t f o r w a r d m o t i o n eventually allows the
base (n) to escape from the danger of exonucleolytic attack.
W h e n S is i n c o r p o r a t e d , there is a p r o b a b i l i t y
qS of going b a c k w a r d s (hydrolysis) and a probab i l i t y I-qS of m o v i n g f o r w a r d ( i n c o r p o r a t i o n ) . I n
ease of i n c o r p o r a t i o n S or A are i n t r o d u c e d
"with respective p r o b a b i l i t i e s pS and pA (pS ÷
pA = I). We suppose for s i m p l i c i t y that the
e n z y m e is r e p l i c a t i n g a h o n m p o l y m e r a n d that pS
a n d pA are i n d e p e n d a n t of the p r e c e d i n g base.
Let us call p s a n d p x the overall f r e q u e n c i e s of
i n c o r p o r a t i o n of S a n d A in the n e w l y synthesized strand. I n the case of the h y d r o l y t i c function of the aminoaeyl-tRNA ligase `we h a d :
ps/pA = pS (I_qS)/[p~t (I_qa)_]
(21)
Now, the expression is more complicated since
the p o l y m e r a s e can move a n u m b e r of steps
ES~
"
Hi
? ES.,-t- Si
(20)
H.,
w h e r e S1 represents the acylated tRNA, S o stauds
for the second a m i n o a e i d and the H's refer to
states of the enzyme i m m e d i a t l y after the occnr e n c e of a hydrolysis. (We are not interested here
i n k n o w i n g what are the substrates that r e m a i n
t e m p o r a r i l y on the enzyme after hydrolysis), The
situation presents a formal s i m i l a r i t y to that of
BIOCH1MIE, 1975, 57, n o 5.
forward, go b a c k w a r d several steps, etc. We have
to c o n s i d e r all possible trajectories of the DNA
p o l y m e r a s e along the template. We have started
s t u d y i n g the p r o b l e m both e x p e r i m e n t a l l y and
theoretically (J. N. e F. Bernardi, w o r k in progress). Our t r e a t m e n t leads to the following
expression :
Enzyme
p s = pS (I_qS)/Ei_K(i.qS)
(22)
where K is the small root of E q u a t i o n (23) :
Kz (I-qS) (I-qa) - - K (I-qSqA) + pSqS + paqA = 0
(23)
Setting :
a s ~- [I-K(I-qA)l/[I-K(I-qS)~
(24)
one gets
ps/pA
= tt SA pS(I_qS)/[pA(I_qX)~
(25)
a s appears as a corrective factor w h i c h may be
called the <<peelback >> corre.ction term ~28q.
Take the l i m i t i n g ease where pS = qS -- I_pA
I-q A, one obtains :
S
~A
~ qA/(I-qA)
(26)
Therefore one can make the peelback term as
i m p o r t a n t as one "wishes. However, i n most practical situations, the peelback t e r m is r a t h e r
u n i m p o r t a n t , and w i l l be neglected in the forthc o m i n g considerations.
The e l e m e n t a r y prc~babilities p's and q's are
related to the e l e m e n t a r y kinetic p a r a m e t e r s (the
k's). Suppose that k s and k A are the M n e t i c
constants for the h y d r o l y s i s of a correct basep a i r a n d the c o r r e s p o n d i n g i n c o r r e c t base-pair
as measured in the absence of synthesis. The
c o n t r i b u t i o n of the h y d r o l y t i c f u n c t i o n to discrim i n a t i o n (I-qS)/(I-qA) may be larger t h a n kA/k s
only if there is a delay in the escape from the
exonucleolytic site. W h e t h e r or not a delayed
escape effect holds ~,ould d e p e n d on the mechan i s m of i n c o r p o r a t i o n at the head of the enzyme ;
that is on the n u m b e r of reversible or i r r e v e r s i b l e
steps involved and the b a l a n c e b e t w e e n the
kinetic p a r a m e t e r s of the p r o p a g a t i o n reaction
and those of the h y d r o l y t i c reaction.
D i s c r i m i n a t i o n b e i n g related to the time w h i c h
separates two i n c o r p o r a t i o n s , it should depend on
the c o n c e n t r a t i o n s of the dNTP's. More precisely,
the errors of in.corporation at a n y p o s i t i o n along
the c h a i n would i n c r e a s e w i t h i n c r e a s i n g c o n e e n trations of that dNTP w h i c h is to be i n c o r p o r a t e d
at the n e x t position. Such an effect can in p r i n ciple be revealed in classically designed experiments [29, 30~.
Practically, the exonuelease activity results in
the c o n v e r s i o n of nueleoside t r i p h o s p h a t e s into
nneleoside m o n o p h o s p h a t e s . If Hopfield's scheme
applies to DNA p o l y m e r i z a t i o n , one should also
be able to observe a release of nucleoside monophosphate, but governed by different laws than
the release due to the exonuclease activity. One
may also w o n d e r "why Hop field's scheme could
not be applied twice, the p o l y m e r a s e cleaving
BIOCHIMIE, 1975, 57, n ° 5.
593
Discrimination.
two phosphates in a row. A c o n s e q u e n c e of a
repeated Hop field scheme for DNA p o l y m e r i zation would be the formation of both n u c l e n s i d e
d i p h o s p h a t e s and nucleoside m o n o p h o s p h a t e s in
the course of the p o l y m e r i z a t i o n reaction. We
have p e r f o r m e d a n u m b e r of e x p e r i m e n t s w i t h
E. c o l t DNA p o l y m e r a s e I, E. c o l t RNA p o l y m e r a s e
and two e u k a r y o t i c DNA polymerases (J. N., F.
Bernardi, G. Brun, A. Assairi, M. L a u b e r a n d F.
Chapeville, m a n u s c i p t in p r e p a r a t i o n ) . In most
cases, we are able to detect a release of nucleoside
diphosphate
and
nucleoside m o n o p h o s p h a t e
w h i c h parallels the i n c o r p o r a t i o n reaction. F o r
instance, u s i n g E. c o i l DNA p o l y m e r a s e I, Poly
(dC) as a template i n the p r e s e n c e of m a n g a n e s e ,
dGTP a n d dATP as c o m p e t i n g substrates, we
followed in parallel e x p e r i m e n t s dGMP i n c o r p o r a t i o n and dGDP and dGMP release a n d also
dAMP m i s i n c o r p o r a t i o n and dADP a n d dAMP
release. We observed a perfect p a r a l l e l i s m
b e t w e e n dGTP c o n s u m p t i o n (resulting m a i n l y in
dGlV[P i n c o r p o r a t i o n ) and dATP c o n s u m p t i o n
( m a i n l y converted into dADP). By <<p a r a l l e l i s m >>,
we m e a n that the c o n d i t i o n Log (UdATP ( t ) ] /
[dATP ( O ) l ) / L o g ([dGTP ( t ) ! / [ d G T P (O)]) =
c o n s t a n t held true t h r o u g h o u t the reaction. Furthermore, the c o n s t a n c y of the ratio [ d A D P ] /
([dAMP i n c o r p o r a t e d ] + [dAMP released]) was
also verified to a very good a p p r o x i m a t i o n . This
a n d other evidence suggest that nueleoside incorp o r a t i o n and c o n v e r s i o n of nucleoside triphosphate into nueleoside d i p h o s p h a t e or monophosphate are alternative outcomes of the i n t e r a c t i o n
of a nucleoside t r i p h o s p h a t e v:ith the polymerasetemplate complex in the course of p o l y m e r i z a t i o n .
They do not prove yet that Hopfield's i n c o r p o r a tion scheme is correct. The possibility r e m a i n s
that nucleoside mono and d i p h o s p h a t e formation
are due to a p a r a s i t i c abortive b r a n c h of the
p o l y m e r i z a t i o n reaction.
DISCUSSION.
The p r o b a b i l i s t i c a p p r o a c h that we have used
a n d the steady-state t r e a t m e n t of enzyme kinetics
are strictly equivalent in their consequences.
T h e y are two `ways of expressing the same basic
p h e n o m e n a . I n that respect, w h e n G o o d m a n e t
al. [28] compute by two different methods errorfrequencies, and then <<prove >>, u s i n g a <<Poisson
model>> that the results are related, they are
merely c h e c k i n g the i n t e r n a l c o n s i s t e n c y of
Mathematics.
The p r o b a b i l i s t i c a p p r o a c h is s i m p l e r w h e n
one is interested in c o m p a r i n g two rates r a t h e r
than in k n o w i n g their absolute values. W i t h the
J. Ninio.
594
steady-state t r e a l m e n t of the Michaelis equation
one w o u l d obtain for i n s t a n c e :
VS __~
IS] [E.] k s k s (k~ + k~)
+ kSO
+
(27)
W h e n m a k i n g the ratio vS/vA, most of the terms
cancel out a n d we are left with just the terms
that we o b t a i n e d directly by p r o b a b i l i t y reasonnings. P r o b a b i l i s t i c equations were developped b y
K n o r r e and Malyguine [20] for c o m p a r i n g the
rates of two reactions o c c u r r i n g on a same
enzyme (for i n s t a n c e the p y r o p h o s p h a t e exchange
r e a c t i o n versus the formation of the a m i n o a c y l a d e n y l a t e on an aminoacyl-tRNA ligasc). However, K n o r r e ' s group did not deal w i t h d i s c r i m i n a t i o n problems.
A n u m b e r of reports, often misleading, a p p e a r e d
r e c e n t l y l i n k i n g a c c u r a c y to kinetics.
Thus, Yarus [31] claimed that <<the very
existence of parallel systems of tRNA a n d synthetase i n the same cytoplasm suppresses misacylalions w h i c h w o u l d otherwise occur >>. Our treatm e n t of the Michaelis scheme should make clear
that u n d e r the c o n d i t i o n of steady-state kinetics,
there is no Yarus-effect to be expected.
Staying w i t h the ligases, a p o i n t of logic can
be made. W h e n the i u c o r r e c t aminoacyl-lRNA
leaves the enzyme, it has a non-negligible probab i l i t y (say 10 p. cent) of going to the ribosome.
Therefore, the e r r o r has to be corrected before
the tRNA leaves the enzyme. H a v i n g a proofr e a d i n g f u n c t i o n for that e r r o r on a n o t h e r ligase
w o u l d b r i n g limited i m p r o v e m e n t s to accuracy.
If there is a n y specificity to be expected i n the
h y d r o l y t i c f u n c t i o n of the ligases, it should be
p r e f e r e n t i a l l y directed t o w a r d s the d e s t r u c t i o n
of the erroneous p r o d u c t s that are made b y the
enzyme itself. T h a t p o i n t seems to have been
overlooked by two i n d e p e n d a n t groups of exper i m e n t a l i s t s [32, 33].
Bessman et at. [13] made e x p e r i m e n t s on the
a c c u r a c y of DNA p o l y m e r i z a t i o n w i t h the T4
enzymes, and checked their e x p e r i m e n t a l results
w i t h a theoretical equation derived by Goodman
et aI. [28]. The authors did not realize that the
<<theoretical equation >> was actually a mere
definition l i n k i n g their e x p e r i m e n t a l q u a n t i t i e s
(their E q u a t i o n (2) can be d i r e c t l y calculated
from t h e i r set of E q u a t i o n s (1)). Thus, even if the
authors had o b t a i n e d their data by d r a w i n g lots,
they w o u l d have been able to check i d e n t i c a l l y
BIOCHIMIE, 1975, 57, n ° 5.
their E q u a t i o n (2). The source of their e r r o r can
be traced back to a confusion i n the theoretical
p a p e r [28]. W h e n w r i t i n g (p. 425) R(Bj) = t u r n
B i / ( i n c Bj + t u r n Bj), the authors identified a
p r o b a b i l i t y of h y d r o l y s i s in the e l e m e n t a r y step
w i t h an overall p r o b a b i l i t y of h y d r o l y s i s i n the
complete process.
It is difficult to judge at the onset w h e t h e r or
not some e n z y m a t i c systems take advantage of the
amplification possibilities that "we have discussed.
F o r one t h i n g our analysis stresses the fact that
a c c u r a c y m a y d e p e n d c r i t i c a l l y u p o n details to
w h i c h no attention w o u l d be paid n o r m a l l y . F o r
instance, a n y analysis of the h y d r o l y t i c activity
in the aminoacyl-tRNA ligases should take into
c o n s i d e r a t i o n the sequence of the events that lead
to the d e ? a r t u r e of the tRNA from the enzyme.
The reaction of d e g r a d a t i o n of ATP described in
E q u a t i o n (8) if observed w o u l d be c o n s i d e r e d as
~'aste, or as due to in vitro artifacts~
One may ask w h y the cell is not w o r k i n g at the
highest achievable level of accuracy. A possible
a n s w e r is that efficiency a n d a c c u r a c y a p p e a r
s o m e h o w a n t i n o m i c . Consider the case of Equation (10). T h e r e is a good d i s c r i m i n a t i o n w h e n
k~ is large c o m p a r e d to k 3 and k_~ a n d k2[$2].
T h e n a c c u r a c y may be controlled t h r o u g h the
c o n c e n t r a t i o n of S 2. By lo:wering it one makes
the reaction more accurate, but at the same time
it appears less e f f i c i e n t : it takes more trials i n
order to have a successful i n c o r p o r a t i o n .
All the reaction schemes that we have consid e r e d involve a last i r r e v e r s i b l e step. T a k i n g
into a c c o u n t the terminal r e v e r s i b i l i t y makes the
analysis far m o r e complicated, but is a necessity
for a valid evaluation of the energetic cost of
accuracy. A p a r t i a l solution to the p r o b l e m m a y
be p r o v i d e d by m a k i n g the last step of the reaction reversible and i n c l u d i n g an a d d i t i o n a l irreversible step w h i c h represents the net consumption of the p r o d u c t by the next step of the metabolic net into w h i c h the c o n s i d e r e d r e a c t i o n is
imbedded.
Acknolwledgments.
I am indebted to L. E. Orgel for the discussions
from which this paper stemmed, and for going patiently through many of its earlier versions. The
work was supported by the Edna MeConnell Clark
Foundation and by the C.N.R.S. (France).
R~sn~r~.
On a 6tudi6 par une approehe probabiliste la relation entre la pr6cision dont un syst6me enzymatique
est capable et le m6canisme r6actionnel. Certains rod-
Enzyme Discrimination.
eanislnes dans lesquels nne des 6tapes est r e t a r d e r
agissent comme des amplificateurs ein6tiques des
d i s c r i m i n a t i o n s mol6culaires. La relation entre notre
schema de r6action retard6e (1) et le sch6ma de
Hopfield (2) est diseut6e.
REFERENCES.
1. Ninio, J. (1975) in <(L'Evolution des Macromoldcures Biologiques )> (C. Sadron, Ed.) Editions du
C.N.R.S., Paris, in the press.
2. Hopfield, J. J. (1974) Proc. Nut. Acad. Sci., USA,
71, 4135-4139.
3. Koshland, D. E. (1958) Proc. Nat. Acad. Sci., USA,
44, 98-104.
4. W o l t e n d e n , R. (1972) Accounts Chem. Res., 5, 1018.
5. Spryer, J. F., Karam, J. D. ,¢ Lenny, A. B. (1966)
Cold Sprin 9 Harbor Syrup. Ouant. Biol., 31,
693-697.
6. Drake, J. W. & Allen, E. F. (1968) Cold Sprinff
Harbor Sgmp. Quant. Biol., 33, 339-344.
7. Freese, E. B. & Freese, E. (1967) Proc. Nat. Acad.
Sci., USA, 67, 650-657.
8. de Vries, F. A. J., S'wart-Idenburg, Ch. J. H. & de
V~Zaard, A. (1972) Molec. Gen. Genet., 117, 60-71.
9. Drake, J. W. (1973) Genetics Supplement, 73, 45-64.
10. Hershfield, M. S. (1973) J. Biol. Chem., 248, 14171423.
11. Nossal, N. G. & Hershfield, M. S. (1973) in DATA
Synthesis in Vitro (Wells, R. D. & Inman, R. B.,
eds.), pp. 47-62, University P a r k Press, Baltimore.
12. Muzyezka, N., Poland, R. L. ~ Bessman, M. J.
(1972) J. Biol. Chem., 247, 7116-7122.
13. Bessman, M. J., Muzyczka, N., Goodman, M. F. z:
Schnaar, R. L. (1974) J. Mol. Biol., 88, 409-421.
BIOCHIMIE, 1975, 57, n ° 5.
595
14. Rosset, R. ,~ Gorini, L. (1969) J. Mol. Biol., 39, 95112.
15. Strigini, P. ~ Gorini, L. (1970) J. Mol. Biol., 47,
517-530.
16. Biswas, D. K. ~ Gorini, L. (1972) J. Mol. Biol.,
64, 119-134.
17. Gorini, L. (1971) Nature New Biol., 234, 261-264.
18. Ninio, J. (1973) Pro9 r. Nucleic Acid Res. Mol. Biol.,
13, 301-337.
19. Ninio, J. (1974) Y. Mol. Biol., 84, 297-313.
20. Knorre, D. G. a Malyguine, E. G. (1972) Dokl. Akad.
Nauk SSSR, 207, 1391-1393.
21. Brutlag, D. ~ Kornberg, A. (1972) J. Biol. Chem.,
247, 241-248.
22. Hamilton, L., Mahler, I. ~ Grossman, L. (1974)
Biochemistry, 13, 1888-1896.
23. Eldred, E. W. ~ Schimmel, R. J. (1972) J. Biol.
Chem., 247, 2961-2964.
24. Yarus, M. (1972) Proc. Nat. Acad. Sci., USA, 69,
1915-1919.
25. Bonnet, J., Gieg~, R. a Ebel, J. P. (1972) FEBS
Letters, 27, 139-144.
26. Yarus, M. a Berg, P. (1969) J. Mol. Biol., 42, 171189.
27. Eldred, E. W. & Sehimmel, P. R. (1972) Biochemistry, 11, 17-23.
28. Goodman, M. F., Gore, W. C., Muzyezka, N. a
Bessman, M. J. (1974) J. Mol. Biol., 88, 423-435.
29. Hall, Z. W. ~ Lehman, I. R. (1968) J. Mol. Biol.,
36, 321-333.
30. Howard, B. D. ~ Tessman, I. (1964) J. Mol. Biol.,
9, 364-371.
31. Yarus, M. (1972) Nature Now Biol., 239, 106-108.
32. Bonnet, J. a Ebel, J. P. (1974) FEBS Letters, 39,
259-262.
33. Sourgoutchov, A., Blanqnet, S., Fayat, G. a Waller,
J. P. (1974) Eur. J. Biochem., 46, 431-438.