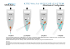Modeling microbial processes in porous media | SpringerLink
Transcription
Modeling microbial processes in porous media | SpringerLink
Modeling microbial processes in porous media
Ellyn M. Murphy 7 Timothy R. Ginn
Abstract The incorporation of microbial processes
into reactive transport models has generally proceeded
along two separate lines of investigation: (1) transport
of bacteria as inert colloids in porous media, and (2)
the biodegradation of dissolved contaminants by a
stationary phase of bacteria. Research over the last
decade has indicated that these processes are closely
linked. This linkage may occur when a change in metabolic activity alters the attachment/detachment rates of
bacteria to surfaces, either promoting or retarding
bacterial transport in a groundwater-contaminant
plume. Changes in metabolic activity, in turn, are
controlled by the time of exposure of the microbes to
electron acceptors/donor and other components
affecting activity. Similarly, metabolic activity can affect
the reversibility of attachment, depending on the residence time of active microbes. Thus, improvements in
quantitative analysis of active subsurface biota necessitate direct linkages between substrate availability,
metabolic activity, growth, and attachment/detachment
rates. This linkage requires both a detailed understanding of the biological processes and robust quantitative representations of these processes that can be
tested experimentally. This paper presents an overview
of current approaches used to represent physicochemical and biological processes in porous media, along
with new conceptual approaches that link metabolic
activity with partitioning of the microorganism between
the aqueous and solid phases.
Received, January 1999
Revised, June 1999, July 1999
Accepted, October 1999
Ellyn M. Murphy (Y)
Interfacial Geochemistry Group, Pacific Northwest National
Laboratory, MSIN K3–61, P.O. Box 999
Richland, Washington 99352, USA
e-mail: ellyn.murphy6pnl.gov
Fax: c1-509-3756954
Timothy R. Ginn
172 Everson Hall, Department of Civil and Environmental
Engineering, University of California Davis
Davis, California 95616–5294, USA
Hydrogeology Journal (2000) 8 : 142–158
Résumé L’introduction des processus microbiologiques dans des modèles de transport réactif a généralement suivi deux voies différentes de recherches: (1) le
transport de bactéries sous forme de colloïdes inertes
en milieu poreux, et (2) la biodégradation de polluants
dissous par une phase stationnaire de bactéries. Les
recherches conduites au cours des dix dernières années
indiquent que ces processus sont intimement liés. Cette
liaison peut intervenir lorsqu’un changement dans
l’activité métabolique modifie les taux de fixation/libération de bactéries des surfaces, soit en facilitant, soit
en retardant le transport bactérien dans le panache de
polluant de l’eau souterraine. Des changements de
l’activité métabolique peuvent en retour être contrôlés
par le temps d’exposition des microbes à des donneurs
ou à des accepteurs d’électrons et à d’autres composés
affectant cette activité. De façon similaire, l’activité
métabolique peut affecter la réversibilité de la fixation,
en fonction du temps de séjour des microbes actifs.
Ainsi, les améliorations de l’analyse quantitative des
organismes souterrains nécessitent d’établir des liens
directs entre les possibilités du substrat, l’activité métabolique, la croissance et les taux de fixation/libération.
Cette liaison nécessite à la fois une compréhension
détaillée des processus biologiques et des représentations quantitatives robustes de ces processus qui puissent être testées expérimentalement. Cet article
présente une revue des approches courantes utilisées
pour représenter les processus physio-chimiques et
biologiques en milieu poreux, en même temps que de
nouvelles approches conceptuelles qui associent l’activité métabolique à la répartition des micro-organismes
entre les phases aqueuse et solide.
Resumen La incorporación de los procesos microbianos
en los modelos de transporte reactivos ha procedido
tradicionalmente a lo largo de dos líneas de investigación independientes: (1) el transporte de bacterias
como coloides inertes en el medio poroso, y (2) la biodegradación de los contaminantes disueltos por una fase
estacionaria de bacterias. En los últimos años se ha
comprobado que estos dos procesos están muy interrelacionados. En concreto, un cambio en la actividad
metabólica puede alterar la relación de adsorción/
desorción de las bacterias, favoreciendo o retardando
el transporte bacteriano en un penacho de contaminaQ Springer-Verlag
143
ción. A su vez, los cambios en la actividad metabólica
están controlados, entre otros factores, por el tiempo
de exposición de los microbios a los receptores/
donantes de electrones. Además, la actividad metabólica puede afectar la reversibilidad de la adsorción, en
función del tiempo de residencia de los microbios
activos. Así, el análisis cuantitativo de la actividad
subsuperficial biótica necesita de un estudio detallado
de las conexiones entre disponibilidad del substrato,
actividad metabólica, crecimiento y relación de adsorción/desorción, lo que requiere tanto un conocimiento
de los procesos biológicos, como una representación
cuantitativa robusta de dichos procesos, comprobable
experimentalmente. Este artículo presenta una revisión
de los métodos actuales para representar los procesos
fisioquímicos y biológicos en el medio poroso, además
de nuevas metodologías para relacionar la actividad
metabólica con el fraccionamiento de los microorganismos entre las fases sólida y acuosa.
Key words contamination 7 bacterial transport 7
numerical modeling 7 microbial processes
Introduction
Progress in modeling microbial processes in porous
media is essential to improving our understanding of
how physical, chemical, and biological processes are
coupled in groundwater and their effect on groundwater-chemistry evolution, bioremediation, and the
reactive transport of contaminants and bacteria. Much
of the emphasis to date has been on quantitative representations of either the kinetics of contaminant degradation or the physical (or physicochemical) processes
that affect the transport of bacteria in porous media,
primarily because these issues are more tractable to the
microbiological and hydrologic transport fields.
Whether the modeling objective is to understand the
biodegradation of contaminants or the movement of
bacteria, the processes that must be considered are the
same. These processes are generally divided into physicochemical and biological. The physicochemical processes include advection, diffusion, dispersion, exclusion, straining, and physical filtration. The physicochemical processes are primarily based on the structure
and properties of the groundwater flow system and
porous media. Consequently, most reactive transport
models incorporate some of the major physical processes, and these processes have been the focus of
numerous experimental and numerical modeling
studies on colloid and biocolloid research. In contrast,
the biological processes of growth/decay, chemotaxis,
predation, physiological adaptation (survival), and
adhesion or active detachment are characteristics of the
bacterial population and by comparison have received
little attention in field-scale hydrogeologic transport
models. Although many researchers readily acknowledge the importance of growth processes in transport
(Harvey et al. 1984; Hornberger et al. 1992; Tan et al.
Hydrogeology Journal (2000) 8 : 142–158
1994), growth is often eliminated in column or field
experiments of biocolloid transport (Champ and
Schroeter 1988; Harvey et al. 1989, 1993; Bales et al.
1995).
Quantitative representations of microbial processes
in saturated porous media are numerous; however, the
coupling of these processes in dynamic contaminant
systems is not well understood. Under oligotrophic
(carbon-limiting) conditions in aquifers, microbial
growth is limited and most of the biomass is associated
with the solid phase (Harvey et al. 1984; Hirsch and
Rades-Rohkohl 1988; Kölbel-Boelke et al. 1988; Godsy
et al. 1992; Albrechtsen 1994). In these growth-limited
environments, physical processes likely dominate transport of that portion of the biomass in the aqueous
phase. In contrast, in nutrient-rich environments, such
as contaminated aquifers, field observations consistently indicate a higher level of biomass in the aqueous
phase. In a contaminated portion of the Cape Cod
aquifer in Massachusetts, USA, Harvey et al. (1984)
report that the aqueous biomass increased by an order
of magnitude, whereas the concentration on the sediments remained approximately the same. Harvey and
Barber (1992) observed 1 30% of total biomass freeliving in a sewage-contaminated plume; Godsy et al.
(1992) note that 90% of total biomass in a creosotecontaminated aquifer was attached, but 49% of
(creosote-degrading) methanogens were in the aqueous
phase. Likewise, at an in-situ bioremediation study at
the Savannah River Site in Georgia, USA, the proportion of methanotrophs, which were stimulated to
degrade chlorinated hydrocarbons, increased by as
much as five orders of magnitude in the aqueous phase
(USDOE 1993). These observations are consistent with
specific recognition of growth-induced partitioning to
the aqueous phase (Jenneman et al. 1985, 1986;
Reynolds et al. 1989; Sharma et al. 1993). Such conditions indicate a greater propensity for transport of
native microbes under natural hydraulic gradients or
under pumping as part of an accelerated bioremediation strategy when growth is a factor.
This article first briefly reviews physicochemical and
biological microbial processes of relevance to subsurface phenomena on short time scales (e.g., bioremediation) and the quantitative representations that have
been developed for these processes. Then, experiments
are discussed that link the physicochemical and biological processes (specifically, growth and attachment of
bacteria) and new approaches for quantitatively
describing these coupled processes in reactive transport
models.
Background on Reactive Transport Modeling
of Coupled Processes
The terminology used in interdisciplinary studies is
sometimes more confusing than the actual processes
involved. Chemical reactions that involve different
phases (e.g., aqueous and solid) are termed heterogeQ Springer-Verlag
144
neous, and reactions that involve a single phase are
termed homogeneous. Unfortunately, aquifer properties that vary spatially are also termed heterogeneous,
whereas properties that are constant in space are
termed homogeneous. Adhesion in this paper refers to
physiologically driven attachment of a microorganism
to a surface, an active process initiated by the microorganism. In this regard, this definition of adhesion is
much narrower than that of others who may include all
intermolecular and surface forces in the definition of
adhesion (for this more generic classification, the term
attachment is used). Partitioning refers to the phase
separation of a microbial community and is equivalent
to attachment/detachment. Partitioning is a general term
that does not denote a specific process or mechanism
that controls the distribution of bacteria between the
mineral and aqueous phases in a flowing system.
Kinetic attachment/detachment rates may arise from
growth or metabolic activity, but they do not vary
between resting and active cells. Dynamic partitioning
refers to a temporal change in the attachment/detachment kinetics as a function of growth activity. Substrate
denotes the energy source/electron donor for biodegradation and does not refer to the solid-phase aquifer
materials.
Reactive transport models are no more than a
collection of process representations (physical, chemical, and biological) of varying accuracy and sophistication that are used to describe a coupled dynamic
system. The relative importance of individual processes
can only be assessed through experimentation and data
collection. More often than not, key processes are
either poorly understood or lumped into a single
expression of mass-transfer rate in reactive transport
models. Consequently, parameter fitting is often
invoked to fit, sometimes non-uniquely, a set of simplified process representations to describe a particular
system. Because isolation of the effects of individual
processes on the overall system dynamics typically
requires sequential data analyses and experimental
design, an iterative theoretical modeling and experimental approach that includes both laboratory and
field studies is expected to yield the greatest advances
in our understanding of these complex, coupled processes.
This section summarizes some of the important
issues in modeling the coupled processes involved in
subsurface microbial activity, ranging from model
construction to model solution. These are treated in
reverse order, beginning with the challenges in solving
fate and transport models involving multiple rates of
reactions, and concluding with broad summaries of the
constitutive theories used to develop mechanistic
expressions for particular processes.
Multiple Rates of Reactions
In the subsequent sections, individual reaction-rate
expressions for various processes involved in subsurHydrogeology Journal (2000) 8 : 142–158
face biotic activity are discussed in some detail. The
equally important issue of simulating a complex set of
these processes within the framework of a conventional
flow and transport model is not the focus of this review
but is relevant and therefore is briefly mentioned
here.
One of the aspects of a reaction system that is most
important for modeling fate and transport in porous
media is the range of the reaction rates. Reacting
mixtures can involve a variety of individual reactions,
each with a particular rate, and the set of rates can
range from the very slow to the very fast to the instantaneous. Instantaneous reactions (that is, transformations that occur relatively instantaneously when viewed
on the time scale of transport processes) involve equilibrium relations between interacting chemicals and are
termed equilibrium reactions. Reactions that involve a
rate that is on the same time scale as transport (that is,
transformations that have a relatively finite rate) are
termed non-equilibrium, or kinetic, reactions. In
general, the majority of subsurface biotic transformations are non-equilibrium and have similar time scales
(e.g., rates within 1–2 orders of magnitude). Because
these rates are similar, these problems are easily
handled with conventional reactive-transport numerical-solution schemes such as operator splitting (e.g.,
Chilakapati et al. 1998).
However, some biotic and chemical transformations,
such as solute adsorption, occur in much shorter time
scales. When this occurs, the reaction rates of the
coupled system may span many orders of magnitude
(e.g., contain both kinetic and equilibrium transformations), and the system must be solved using increasingly
smaller time steps over a longer time frame to capture
the effects of the fast reactions upon the slower kinetic
and transport transformations. Such systems are difficult to simulate because the short time step required to
accurately capture the fast reactions can render the
computational costs prohibitively expensive. One
approach to such systems is to treat the “fast” reactions
as occurring instantaneously, i.e., as equilibrium transformations. The resulting model description contains a
combination of differential and algebraic equations,
i.e., a “DAE system.” Specialized approaches for
numerically solving reactive transport systems under
both fully kinetic formulations (using operator splitting) and mixed kinetic-equilibrium formulations (using
specialized DAE solvers such as DASSL; Petzold 1983)
are available in the public codes HYDROGEOCHEM
(Yeh and Tripathi 1989) and RAFT (Chilakapati 1995).
The reader is referred to a more thorough review of the
issue of reaction systems in Steefel and MacQuarrie
(1996).
Structure of Subsurface Biomass
The constitutive theory used to upscale the processes
occurring at the cell scale (to the degree that they are
understood) to the bulk-phase scale depends on the
Q Springer-Verlag
145
nature of the various reactions involved in subsurface
biotic processes, including their speeds and reversibility. The description of these cell-scale reactions is
highly dependent on the structure of the biological
phase. The basic representation of the structure of a
biological phase in porous media, however, is a matter
without consensus, as noted in the exchanges of Baveye
and Valocchi (1991), Widdowson (1991), Baveye et al.
(1992), and Jaffe and Taylor (1992). Typically, in structured biomass models, the biophase is represented as
either a (1) continuous biofilm on the solid surface
(Taylor and Jaffe 1990; Taylor et al. 1990), or (2)
discontinuous patchy film (Widdowson et al. 1988;
Vandevivere and Baveye 1992; Rittman 1993). Mathematically, such structured biofilm models are often
associated with a diffusion limitation on the transport
of solutes from aqueous phase to biomass phase, where
they can be degraded (Wood et al. 1994).
In the so-called unstructured biomass models, no
assumptions are made on the biophase structure
(MacQuarrie et al. 1990; Sudicky et al. 1990; Zysset et
al. 1994), and the biomass is treated as a suspended but
kinetically sorbing/desorbing species (MacQuarrie et al.
1990; Zysset et al. 1994). Therefore, in unstructured
models the biomass is a fully penetrable volumeless
component that assumes that a linear relation exists
between mass of substrate consumed and mass of
biomass produced and that no diffusion limitations
affect the transfer of substrate mass from solution into
the biomass. This approach has been taken in past
column studies that focus on bacterial transport
(Lindqvist et al. 1994; Tan et al. 1994) and in studies of
intermediate-scale flow cells that focus on active
degradation and growth and coupled transport
(Murphy et al. 1997b).
The degree to which the particular structural
assumption impacts the resulting expressions for reaction transformation is incompletely understood. For
example, the process of metabolic lag (Wood et al.
1995), discussed below, may be experimentally indistinguishable from a substrate diffusion limitation through
a biofilm. Both processes result in a delay in the onset
of degradation, and the correct labeling of the process
may or may not have an effect on the ultimate amount
of contaminant degraded. Recently, several studies
have used volume averaging to formally upscale the
processes of mass transport and reactions in biofilms
(Wood and Whitaker 1998, 1999, in press). Continuing
investigations such as these may help to refine our
understanding of the role of biofilm structure in the
subsurface (Characklis and Marshall 1990).
Physicochemical Processes
Most reactive transport models that consider microbial
processes incorporate physicochemical processes, such
as advection, dispersion, straining, and physical filtration. Unlike the biological processes, physicochemical
processes affecting microbial transport have been the
Hydrogeology Journal (2000) 8 : 142–158
focus of numerous experimental and numerical
modeling studies. These important processes provide
the framework for bacterial transport and reaction in
porous media. Indeed, the impact of biological processes in a flowing groundwater system can only be evaluated within this physicochemical framework. Therefore, the physicochemical processes are defined and
briefly reviewed in this section, and descriptions are
summarized in Table 1. Readers are referred to reviews
by McDowell-Boyer et al. (1986) and Harvey (1991) for
more thorough discussions of physicochemical processes.
Microbes undergo convective transport as a particulate or a dissolved species moving with the porewater
whose velocity is governed by the hydraulic pressure
gradient, porosity, and permeability distribution
[Table 1, Eqs. (1) and (2)]. The occurrence of nutrient
and/or electron acceptor as a solute undergoing transport may be coupled to the transport process through
the effects of these constituents on the fluid properties
of density and viscosity. Convective transport in porous
media is also associated with hydrodynamic dispersion,
an enhanced mixing process arising from the tortuosity
of the convective paths compounded by molecularscale (diffusional) mixing. The resulting convectivedispersive transport terms are shown in Eq. (3) of
Table 1.
Straining and physical filtration represent the
removal of microbes from solution by physical
(geometric
and
intermolecular/surface)
forces.
Straining is the trapping of microbes in pore throats
that are too small to allow passage and is exclusively a
result of pore geometry (Corapcioglu and Haridas
1984). Prediction of mass removal by straining, based
on purely geometric relations between the effective
diameter of biocolloids and the diameter and packing
(coordination number) of grains, is not significant
where the colloid diameter is less than 5% of the
porous-medium grain diameter (Sakthivadivel 1966,
1969; Herzig et al. 1970; Corapcioglu and Haridas 1984;
McDowell-Boyer et al. 1986; Harvey and Garabedian
1991).
Physical filtration is the removal of particle mass
from solution via collision with and deposition on the
porous medium; here, the term includes both sedimentation [Table 1, Eq. (4)] and attachment [Table 1,
Eqs. (5) and (6)]. Sedimentation is filtration due to
gravity (Corapcioglu and Haridas 1984; McDowellBoyer et al. 1986) and depends on particle buoyancy
(Wan et al. 1995). Many natural bacteria and viruses
are neutrally buoyant, in which case sedimentation is
negligible. However, cultured microorganisms are typically larger and sometimes denser than their native
counterparts (Harvey et al. 1997) and may involve sizeable buoyancy-driven filtration.
Physical forces resulting in attachment (Brownian,
electrostatic, van der Waals, and pore-water hydrodynamic) are the dominant mechanisms for partitioning
of biocolloids to solid media and have received
Q Springer-Verlag
146
Table 1 Quantitative representations of physicochemical processes
Description of process
Quantitative representation
Limitations
Flow: groundwater velocity V(x) is
related to pressure gradient through
Darcy’s law (1) and is governed by
water mass conservation law (2), with
water source q(x). Fluid density r and
viscosity m are assumed constant.
1. uV(x)pPK=h(x)
Unknown multiscale variability of
permeability incurs unknown
non-uniformities in convections.
Transport: solutes and microbes
undergo convective and dispersive mass
fluxes, expressed in partial mass-balance
terms (3) for concentration C of arbitrary (subscript i) species.
Convective transport at groundwater
velocity V may be augmented by microorganism density-induced sedimentation
velocity, ns (for cultured microbes;
Harvey et al. 1997), approximated here
by Stokes’ law for descent of a sphere
of density rs and diameter ds, and
viscosity m (4).
Physical microbial attachment/detachment processes: physical partitioning of
microbes between aqueuos (5) and
attached (6) phases is quasi-empirically
given by various first- or second-order
models (first-order shown here). Cmm
and Cim equal concentration of mobile
microbes and immobile microbes,
respectively. When physical processes
are dominated by passive colloid filtration (7) (Rajagopalan and Tien 1976),
Kf in (8) includes sticking (a) and collision (h) factors that depend on v and
particle properties, and Krp0. In (9),
site saturation limits attachment of
bacteria; where C im
max is maximum retention capacity of saturable sites.
2. =7(uV(x))pq(x)
3.
iCi
it
)
pP=7(Ci(Vcvsẑ)c=7[D7=(Ci)]
transport
4. vs p
(rsPr)gds
18m
5.
iCmm
it
)
6.
iCim
it
phys. part.
7.
iCim
it
8. Kf p
9.
)
)
phys. part.
Fails to capture pore-scale instabilities arising from density/
viscosity, which can greatly
increase mixing and thus biodegradation.
pPKf CmmcKrCim
pKf CmmPKrCim
See clarification of h, which
appears here in Eq. (8), in Logan
et al. (1995).
3 (1Pu)
ah
2 dc
)
pPKf
phys. part. in
saturable sites.
substantial attention, partly as a result of their quantitative tractability (cf. review by McDowell-Boyer et al.
1986). A typical model is shown in Table 1, Eqs. (5) and
(6). The microbe is treated as a spherical particle
moving through a uniformly packed homogeneous bed
of spherical grains (Herzig et al. 1970; Shaw 1970; Tien
et al. 1979). The microbial mass removal from the
aqueous phase has also been cast in terms of porewater velocity, viscosity, and density; media grain size;
and media porosity [Table 1, Eq. (7)]. This approach of
colloid-filtration theory incorporates a sticking coefficient (a) and collision factor (h) in the forward attachment rate [Kf; Table 1, Eqs. (7) and (8)]. The resulting
relations are well known (de Marsily 1986) and have
been widely applied to microbial transport (Harvey et
al. 1989; Harvey and Garabedian 1991).
Previous research has demonstrated that attachment
is influenced by (1) solution ionic strength through the
effect on electrostatic interactions (Sharma et al. 1985;
van Loosdrecht et al. 1989; Scholl et al. 1990;
McDowell-Boyer 1992; Shonnard et al. 1994; Tan et al.
1994); (2) pH (McEldowney and Fletcher 1988); and
(3) mineralogy (Fletcher and Loeb 1979; Scholl et al.
Hydrogeology Journal (2000) 8 : 142–158
Non-mechanistic, essentially
empirical model fails to capture
potentially important cause-effect
relations for microorganisms
actively involved in biodegradation.
pKf vCmm
phys. part.
iCmm
it
Unknown non-uniformities in
convective water flux complicate
tracking of solution substrates,
electron acceptors, and microbes.
(C max
im PCim)
CmmcKrCim
C max
im
1990; Mills et al. 1994). The major mineral component
of most aquifers, quartz, is predominantly negatively
charged, as are most bacteria; thus, the hydrodynamic
and attractive forces must overcome the repulsive electrostatic force for bacterial immobilization to occur.
Sand grains coated with iron hydroxide have positive
surface charges, thus reversing the electrostatic force
from repulsive to attractive and increasing the likelihood of microbial attachment. Hydrophobic interactions can also result in sorption of microorganisms
(Fletcher and Loeb 1979; van Loosdrecht et al. 1987;
Fletcher 1991; McCaulou et al. 1994). The reversibility
of physical filtration, via reduction in solute ionic
strength (Scholl et al. 1990; McDowell-Boyer 1992;
Bales et al. 1995), is not inherent in models based on
filtration theory, because the filtration models represent irreversible deposition only under conditions of
uniform flow direction and fixed solution chemistry.
Thus, treatment of detachment is entirely absent in
several filtration-theory analyses of microbial transport
(e.g., Jewett et al. 1995). The evidential significance of
detachment processes in experimental studies,
however, has led to incorporation of a more-or-less
Q Springer-Verlag
147
empirical detachment term. The resulting models use
some combination of sites undergoing equilibrium
attachment; sites undergoing irreversible kinetic attachment, in accordance with filtration theory; and sites
undergoing kinetic-reversible attachment (Bales et al.
1991; Harvey and Garabedian 1991; Lindqvist and
Bengtsson 1991; Mills et al. 1991; Hornberger et al.
1992; Kinoshita et al. 1993; McCaulou et al. 1994).
Some researchers suggest augmenting the first-order
kinetic attachment model with a non-linear governing
factor intended to represent the attachment-limiting
effect of site saturation at saturable sites [Table 1,
Eq. (9)], while maintaining a linear attachment rate at
other sites and a linear detachment rate overall (Lindqvist et al. 1994; Tan et al. 1994; McCaulou et al. 1995;
Saiers and Hornberger 1996), or a residence-time
controlled detachment rate (Johnson et al. 1995).
In addition to physical filtration, size exclusion
results in differential bacterial and ion-tracer breakthrough times in column (Hornberger et al. 1992;
Mayotte et al. 1996) and field experiments (Wood and
Ehrlich 1978; Pyle and Thorpe 1981; Harvey et al.
1989). Size exclusion is the phenomenon of transported
particles moving faster than the pore water, or at least
faster than the average pore-water velocity, as indicated by the breakthrough of an inert molecular-scale
tracer. Pore-water velocity within a capillary or pore
throat is generally parabolically distributed, in which
the maximum velocity occurs at the centerline and
velocity at the pore walls is equal to zero (de Marsily
1986). Conventional transport theory assumes that
molecular-scale solutes thoroughly sample the full
distribution of velocities. Microbes and large colloids,
by virtue of their size, preferentially experience the
higher velocities near pore centerlines, yielding an
average velocity that is higher than that of a dissolved
tracer. Thus, microbes can precede the tracers downgradient. The occurrence of exclusion typically requires
the bacterial diameter to be ~1% of the porous
medium-grain diameter, which is common for transport
in sandy aquifers (Dodds 1982; de Marsily 1986). When
the electrostatic forces between the media and colloid
are repulsive, as is the case with negatively charged
microbes in negatively charged quartzitic media, the
force field tends to channel the microbes closer to the
pore-throat centerlines and away from the walls (anion
exclusion; de Marsily 1986). Thus, the effect may be
drastically more pronounced at larger observation
scales in natural media, as has been reported in some
experiments (Pyle 1979; Enfield and Bengtsson 1988;
Harvey et al. 1989, 1993; Shonnard et al. 1994).
Biological Processes
Growth and decay processes are generally linked to
spatial and temporal variations in nutrient flux through
Monod (substrate-limited) or dual-Monod (substrate
and electron-acceptor limited) microbial reaction kinetics (Monod 1949). Several forms of Monod-based
Hydrogeology Journal (2000) 8 : 142–158
kinetic equations are used for modeling different types
of microbial metabolisms (Molz et al. 1986; Widdowson
et al. 1988; Kindred and Celia 1989; Taylor and Jaffe
1990; Kinzelbach et al. 1991; Wood et al. 1994; Zysset et
al. 1994; Ginn et al. 1995; Corapcioglu and Kim 1996;
Koch 1998).
For example, Table 2 shows the evolution equations
for a solute [Eq. (1)] undergoing aerobic degradation
with consumption of electron acceptor [oxygen;
Eq. (2)]. The concurrent growth of aqueous and
attached biomass is shown in Eqs. (3) and (4) of Table 2
(Murphy et al. 1997b). Such studies account for
biomass growth through a simple linear conversion of
mass of nutrient degraded to biomass increase [see the
factors F and Y in Eqs. (1) and (2), where F is the mass
ratio of electron acceptor per substrate consumed, and
Y is the yield coefficient or biomass per mass
substrate]. Monod kinetics generally work well for
bacterial populations having low saturation constants
for organic substrates, as is normally the case in subsurface environments (Harvey and Widdowson 1992). The
Monod formulation was originally based on MichaelisMenten enzyme kinetics, and the Monod coefficients
and formulation itself are quasi-empirical (Button
1993).
Several enhancements have been incorporated into
Monod kinetics to address limitations in the original
formulation. For example, the Monod formulation
represents growth rate as depending only on the instantaneous concentration of substrate and electronacceptor and does not account for a lag in the response
of growth rate to changes in substrate concentration,
nor does it account for the historical variations in
substrate concentration (Powell 1967). Metabolic lag is
essentially the delay in biodegradation of a contaminant between the time that the contaminant is first
encountered and when it is utilized. This delay generally results from the time it takes to synthesize enzymes
necessary to take up or metabolize the contaminant.
Degradation rates in natural media may reflect
different levels of microbial metabolic activity, which
depend on the history of nutrient availability to the
microorganism and on the history of the growth of the
microorganism (e.g., Wood et al. 1995). Different
approaches to accounting for the resulting lag in microbial degradation under a change from nutrient-limiting
to nutrient-rich conditions are described in Wood et al.
(1995) and in Ginn (1999). The Wood et al. (1995)
formulation [Table 2, Eq. (5)] is based on the threshold
concentrations of substrate and electron acceptor,
which can be experimentally determined. This formulation works quite well for attached microbial populations; however, when the microbes partition between
phases, their metabolic potential arises as a distributed
quantity, which is accounted for by using the approach
of Ginn (1999), described below.
Endogenous respiration is the process by which
microorganisms consume cell reserves in the absence of
substrate and thereby continue to use a terminal elecQ Springer-Verlag
148
Table 2 Quantitative representations of biological processes
Description of process
Biodegradation: solution substrates
(Cc) and electron acceptors (C0)
undergo transformations by both
aqueous microbes (Cmm) and
attached microbes (Cim). In simplest
case, transformations of substrate (1)
and electron acceptor (2) are limited
by nutrient availability expressed by
Monod {bracketed} factors. Ypyield
coefficient (biomass/mass substrate)
and Fpelectron acceptor/mass
substrate.
Microbial growth: substrate and electron acceptor degradation induces
changes in biomass of both aqueous
(Cmm) and attached (Cim) microbes;
mMpspecific growth rate.
Metabolic lag (l) is the delay
between time when a microbe first
encounters an electron donor and
when it is able to build the enzyme
systems required to use the electron
donor.
Endogenous respiration (b0) is the
process where microbes consume cell
reserves in absence of donor and
continue to use an electron acceptor.
Random motility and chemotaxis are
microbial transport fluxes driven by
both diffusion-like random motions
and automobility (vx) directed
toward increasing substrate concentrations (upgradient; e.g., Barton and
Ford 1995). dm is the random
motility coefficient.
Competitive inhibition occurs in
mixed populations that use the same
nutrients (Bailey and Ollis 1986;
Semprini et al. 1991), where CI is
inhibitor concentration and KI is
inhibition constant.
Cometabolism is the transformation
of a compound that does not yield
energy or growth. C2 is concentration of non-growth contaminant; k2/
K2 is a ratio of constants equivalent
to second-order rate constant.
Quantitative representation
iCc
it
)
iC
2.
it )
1.
pP
bio deg’n
Cc
lmM
C0
(CmmcCim)7
Y
(K0cC0) (KccCc)
0
pPF
bio deg’n
3.
4.
iCmm
it
)
iCim
it
bio deg’n
5
5
Cc
C0
0
0
c
0
pPlmMCim
0
c
c
0
c
c
t
Interaction with diffusive
processes unknown, i.e., what
is threshold level of substrate
required for induction?
Unable to incorporate with
partitioning microbes.
5. l(t)p # K(t)CS(tPt) dt
0
terms described in Wood et al. (1995)
6.
7.
8.
9.
iC0
it
)
pPb0(CmmcCim)7
endo. resp.
iCmm
it
)
This representation lumps
both (static) baseline maintenance with endogenous respiration.
Endogenous respiration is
dynamic and likely depends
on concentration of storage
reserves.
0
Chemotactic motility models
are quasi-empirical and
mainly fitted to monoclonal
cultured populations – results
for natural environment
strains are few.
p=7(dm=CmmPCmmvx)
motility
k2
iC2
p P(CmmcCim)
it
K2
3
Cc
CI
KccCc c
KI
C0
4 3K cC 4
0
0
Invariance of inhibition
constant requires steady-state
assumption for competitive
population.
C2
1 21 C cK 2
tron acceptor (TEA). The term maintenance respiration
usually refers to a baseline respiration rate in the presence of substrate that provides cell energetic requirements for survival or preservation of a particular cell
state, which is not associated with growth (Bailey and
Ollis 1986; Beeftink et al. 1990). This distinction
Hydrogeology Journal (2000) 8 : 142–158
C0
5 (K cC ) 6
0
lmM
iCc
pP
(CmmcCim)
it
Y
6
No mechanistic connection
between growth and microbial
detachment.
During growth-mediated
transport, microorganisms
enter aqueous stream as a
result of cell division.
5 (K cC )(K cC ) 6
C
C
75
(K cC ) (K cC ) 6
pPlmMCmm7
Difficult to distinguish kinetic
rates for attached and unattached microorganisms.
6
Cc
lmM
C0
(CmmcCim)7
Y
(K0cC0) (KccCc)
bio deg’n
)
Limitations
2
2
between endogenous respiration and maintenance
energy is not universally accepted (Herbert 1958; Pirt
1975; Smith et al. 1986; Smith 1989; Hess et al. 1996).
Although maintenance energy is important and highly
relevant to questions regarding the long-term survival
of microorganisms in oligotrophic environments, it is
Q Springer-Verlag
149
probably less important than endogenous respiration in
the description of biodegradation and microbial transport in a dynamically evolving contaminant plume.
Subsurface microorganisms may have highly variable endogenous respiration rates (Novitsky and
Morita 1977) that depend directly on nutrient exposure
history and hence the level of cell reserves. The cell
reserves are highest after a sustained growth phase, and
even in the absence of contaminant degradation, the
terminal electron acceptor continues to be depleted.
Therefore, endogenous respiration affects the redox
conditions of the groundwater long after the substrate
has disappeared (Murphy et al. 1997b). Experimental
evidence suggests that the initial period of starvation,
after substrate disappearance, is characterized by an
increase in endogenous respiration (Kjelleberg et al.
1987), possibly due to production of starvation proteins
(Smigielski et al. 1989; Matin 1990; Oliver et al. 1991).
An increase in cell division has also been noted at the
onset of starvation (Novitsky and Morita 1976) and
may be a survival response to increase the surface-tovolume ratio of the cell. Sometime after the onset of
starvation, endogenous respiration sharply decreases
and these minimal rates may be associated with a
dormancy phase (Novitsky and Morita 1977; Kaprelyants et al. 1993). Under these conditions, respiration
may only be used to maintain basic cell structures and
repair DNA. The rate of endogenous respiration can be
highly variable, yet in biodegradation models endogenous respiration is often combined with maintenance
respiration and usually treated as a constant parameter
rather than a dynamic process linked to cell reserves
and physiological state. A typical quantitative formulation is shown in Table 2, Eq. (6). Mechanistic formulations of dynamic endogenous respiration based on both
nutrient history (e.g., cell reserves) and threshold electron donor/acceptor concentrations would require an
accounting of the time a microorganism has been
exposed to nutrients, a capability lacking in current
modeling approaches.
Several modeling studies have ignored the explicit
presence of bacteria in both aqueous and attached
phases and their dual role in contaminant removal
(Molz et al. 1986; MacQuarrie et al. 1990; Chen et al.
1992). A few studies have considered the presence of
cells at various phases, but they have also assumed
microbial reaction kinetics to be independent of the
phase in which cells reside (Taylor and Jaffe 1990;
Zysset et al. 1994; Corapcioglu and Kim 1996; Murphy
et al. 1997b). This assumption may not be adequate,
because cells attached to the solid phase may behave
differently from the cells suspended in the aqueous
phase. Harms and Zehnder (1994) provide data indicating that attached microbes degrade substrate more
slowly than their aqueous-phase counterparts, and they
attribute the difference to limitations on substrate
transport by diffusion to the cell surface due to the
presence of the solid phase. Eisenmann et al. (1998)
report that the rate of predation of aqueous bacteria
Hydrogeology Journal (2000) 8 : 142–158
was twice the rate of attached bacteria and that the
rates were further halved in a flowing system. Further
modeling studies supported by experimental evidence
are needed before general conclusions can be made about
phase-dependent microbial reactions in porous media.
Additional biological processes affecting microbial
transport are expressed through the growth/decay
process and include active adhesion/detachment,
survival, and chemotaxis. Active adhesion/detachment
is treated here as a biological-driven process. Several
studies report that microorganisms exhibit active adhesion/detachment processes that may be a response to
local nutrient availability (Dawson et al. 1981; Kjelleberg and Hermansson 1984; van Loosdrecht et al.
1990), survival mechanisms (Dawson et al. 1981;
Wrangstadh et al. 1990; Gilbert and Brown 1995), and/
or growth (Jenneman et al. 1985, 1986; Reynolds et al.
1989; Sharma et al. 1993). No generally accepted quantitative treatment of active adhesion/detachment processes exists. The distinctions between a microorganism’s response to nutrient availability, survival
stress, and growth are not necessarily separable nor are
they independent processes.
Microorganisms that have the capability to move in
response to a chemical gradient are termed chemotactic. Both taxis (possessing motility genes) and
chemotaxis have been cited as potential means of transport for subsurface organisms (Corapcioglu and
Haridas 1984; Jenneman et al. 1985; Reynolds et al.
1989; Mercer et al. 1993; Barton and Ford 1995). Quantitatively, taxis is an effective diffusive flux for microorganisms that depends on the local spatial gradient in
aqueous microorganism concentration, and chemotaxis
is a flux of microorganisms associated with the gradient
in nutrient supply. These two terms are shown in order
in Table 2, Eq. (7). Chemotaxis requires energy and
therefore is closely linked to growth processes in
porous media. In oligotrophic environments, nutrient
gradients are quite small and are likely to be associated
with either preferential flow paths (if the nutrients arise
from recharge) or solid-phase chemical heterogeneity.
Chemotaxis may be a very important transport mechanism in these low-nutrient environments. Mercer et al.
(1993) observed that bacteria subjected to oligotrophic
conditions displayed enhanced chemotactic response.
A contaminant plume results in large chemical
gradients that may also contribute to microbial transport via chemotaxis. Like virtually all microbial characteristics, tactic capability varies widely among organisms. Therefore, these organism-specific transport characteristics have not been incorporated into predictive
models of microbial transport applicable to field-scale
hydrogeological applications. However, much work has
been done on developing basic models of chemotactic
transport of cell populations in response to gradients in
aqueous-phase nutrients. These efforts and the
resulting models are beyond the current scope of this
paper; the interested reader is referred to the review of
Ford and Cummings (1998).
Q Springer-Verlag
150
Several other processes become important when
analyzing multiple interacting microbial populations.
Population interactions that have received the most
attention are competition, predation, and cometabolism (Bailey and Ollis 1986; Semprini et al. 1991;
Mohn and Tiedje 1992; Semprini and McCarty 1992;
Harvey et al. 1995; Lang et al. 1997; Smith and McCarty
1997; Smith et al. 1997). Although competition has a
much broader definition in population dynamics, in
terms of representing this process in Monod kinetics,
competition is simply when two or more microbial
species compete for the same nutrients. A Monod
formulation for competitive inhibition is shown in
Table 2, Eq. (8). Predation, primarily by protozoa,
affects a microorganism’s ability to survive and may
also be a crucial process controlling aqueous-phase
biomass concentrations in groundwater (Harvey et al.
1995). Cometabolism is the transformation of a
compound by a microorganism that is incapable of
using the compound as a source of energy or growth
[Table 2, Eq. (9)]. Generally, cometabolism occurs in
the presence of a growth substrate or other transformable compound, but it also may include transformations
by resting cells if no growth occurs (Chang et al. 1993;
Criddle 1993; Smith and McCarty 1997). In one of the
most common examples of cometabolism, aerobic
bacteria employ oxygenases, such as methane monooxygenase in methanotrophic bacteria, to oxidize chlorinated solvents (Little et al. 1988; Mohn and Tiedje
1992; Ely et al. 1997; Smith and McCarty 1997; Smith et
al. 1997).
Metabolic Effects on Microbial Transport
and Contaminant Degradation
Modeling studies often simplify the explicit presence of
bacteria in both aqueous and attached phases, instead
treating the biomass as a fixed, often uniform phase. In
reality, bacteria are distributed both in the aqueous and
on the solid phases, and this distribution is dynamic in
the presence of a contaminant plume. In an experiment
conducted in an intermediate-scale flow cell
(100!20!10 cm dimensions), a substrate pulse
resulted in an increase in aqueous-phase bacteria, as
shown in Figure 1 (Murphy et al. 1997b), similar to
observations in field bioremediation efforts (USDOE
1993). Subsequent column experiments suggest that
this response may be cell-division-mediated transport, a
mechanism long recognized in the microbiology literature (Kjelleberg et al. 1982; Jenneman et al. 1985, 1986;
Reynolds et al. 1989; Sharma et al. 1993). Cell-divisionmediated transport has also been referred to as
mother–daughter or shedding cells and occurs when the
“mother” cell, attached perpendicular to the mineral
surface, grows and divides. The “daughter” cell is
released into the aqueous phase (Marshall 1996) and
the mother cell remains attached.
Hydrogeology Journal (2000) 8 : 142–158
Figure 1 Breakthrough curve of biomass in response to a pulse
of substrate in an intermediate flow cell. Flow cell was packed
with sand equilibrated with P. cepacia sp. 866A. AODC
Acridine orange direct counts. (After Murphy et al. 1997b)
The aqueous-phase partitioning of bacteria in
response to cell division was investigated in sandcolumn experiments where cell division was blocked in
one column by nalidixic acid, an antibiotic that
prevents DNA replication. Results are shown in
Figure 2. When cell division was blocked, no increase
occurred in the aqueous-phase bacteria (Pseudomonas
cepacia sp.), whereas a characteristic increase was
observed in the control column that did not contain
nalidixic acid (Murphy et al. 1997a). Collectively, this
experimental information suggests that a strong
coupling exists between metabolic processes and
aqueous partitioning or transport of the microbial
community.
As discussed above, many investigators note that
starvation or nutrient availability can stimulate a
change in the partitioning of a microbial community
Figure 2 Breakthrough of biomass in columns packed with sand
equilibrated with P. cepacia sp. 866A in response to a substrate
pulse. In one column, cell division was inhibited by maintaining
a constant level of nalidixic acid. AODC Acridine orange direct
counts
Q Springer-Verlag
151
between the solid and aqueous phases. A contaminant
plume creates a dynamic nutrient environment, but it is
not clear whether the corresponding response in partitioning of the microbial community has any effect at all
on the actual contaminant degradation. Therefore,
Ginn et al. (1998) investigated the relative importance
of dynamic partitioning of the bacterial phase on
contaminant degradation by modeling the response of a
consortium of anaerobic bacteria involved in the
degradation of chlorinated hydrocarbons. This consortium consisted of two organisms, a propionate degrader
that produces formate and displays dynamic partitioning, and Desulfomonile tiedjei that uses formate and
reductively dechlorinates the chlorinated hydrocarbons. D. tiedjei displays only kinetic partitioning and is,
in general, irreversibly attached. This example concerns
the stimulation of a natural subsurface microbial
community that would be, under initial conditions,
dominantly associated with the mineral phase.
However, when substrate is present, as in a contaminant plume, the propionate degrader displays dynamic
partitioning, e.g., the forward attachment rate, Kf,
changes with the level of metabolic activity. Two examples were compared to determine the effect of the
dynamic attachment/detachment of the propionate
degrader: (1) both bacteria controlled by kinetic attachment/detachment rates, i.e., no change in the attachment/detachment rates with metabolic activity; and (2)
the propionate degrader displays dynamic attachment/
detachment, whereas D. tiedjei continues to display
only kinetic attachment/detachment rates. Kinetic
attachment/detachment rates were formulated as
shown in Eqs. (5) and (6) in Table 1. Dynamic partitioning rates were formulated by allowing the forward
attachment rate to decrease with increasing metabolic
activity, shown here for the case of aqueous microorganisms:
iCmm
c =7(CmmV)p=7[D=Cmm]
it
Cc
Cc
PKf Cmm 1 P
cmMCmm
KscCs
KccCc
3
4
1 3
n
42 cK C
r
im
(1)
where Cmmpconcentration of aqueous “mobile
microbes” (mass per unit pore volume), Ccpconcentration of substrate (mass per unit pore volume),
Cimpconcentration of attached “immobile microbes”
(mass per unit pore volume), Dpdispersion tensor
Vppore-water
velocity
(LT –1),
Kf,
(L 2T –1),
Krpforward and reverse attachment/detachment rates
(T –1), mMpMonod specific growth rate (T –1), and
KcpMonod half-saturation constant (T –1).
In this modeling exercise, a pulse (or plume) of chlorinated hydrocarbon was injected into the left-hand
side of the flow cell, shown in Figure 3. The sediments
in the flow cell consisted of a darker, high-permeability
region, and a lighter, low-permeability region. The
contaminant showed an early breakthrough in the highpermeability portion of the sediment, followed by a
secondary peak of the contaminant moving through the
lower permeability zone (Figure 3a) when both bacteria
Figure 3 Movement and
degradation of a hypothetical
chlorinated hydrocarbon
plume represented by light
area moving from left to right.
Reductive dehalogenation
occurs with a consortium of
bacteria, a propionate
degrader and Desulfomonile
tiedjei. a Bacteria display
kinetic attachment/detachment, or b propionatedegrader displays dynamic
attachment/detachment while
D. tiedjei displays kinetic
attachment/detachment
Hydrogeology Journal (2000) 8 : 142–158
Q Springer-Verlag
152
were only displaying kinetic attachment/detachment.
However, when the propionate-degrader undergoes
dynamic partitioning (Figure 3b), only contaminant
traveling through the high-permeability zone reaches
the end of the flow cell; contaminant moving through
the low-permeability region is completely degraded, as
shown in the 40-h simulation. The enhanced degradation under dynamic conditions is due to the aqueous
partitioning of the propionate-degrader that results in
an increasing population moving with the plume, and
hence increasing concentrations of formate, as the
contaminant plume moves along the flow path. In this
example, the rate of formate production by the
propionate-degrader was limiting the metabolic activity
of D. tiedjei that promotes the dechlorination reaction.
This simulation illustrates the importance of understanding the partitioning of bacteria under dynamic
growth conditions and of being able to track the transient movement of bacteria under changing chemical
conditions.
Exposure Time Model for Tracking a Dynamic
Bacterial Population
One instance of dynamic partitioning occurs when the
propensity for a microorganism to become irreversibly
attached to a solid phase depends on the residence time
of the microorganism near the mineral surface. Residence time is defined here as the amount of time a
microorganism is reversibly associated with a surface
through a specific interaction, such as electrostatic, van
der Waals, or hydrophobic interactions. Irreversible
attachment is usually associated with active adhesion
processes on the part of the microbe (Rijnaarts et al.
1993; Fletcher 1996). For instance, a microbe may
exhibit slow (relative to transport) cell-surface changes,
such as exopolysaccharide production (Williams and
Fletcher 1996; Jucker et al. 1997) associated with
biofilm formation that effectively increases the probability of irreversible attachment over a population of
microbes. Conventional descriptions of partitioning
kinetics at the bulk-phase scale are incapable of
capturing this behavior, because such models cannot
track the distribution of biomass over the contiguous
residence time. This limitation is noted in Johnson et al.
(1995), who provide a heuristic accounting of the
effects of residence time on reversibility by zeroing the
detachment rate for microbes whose residence time
exceeds a particular threshold. A new theoretical
approach allows the tracking of residence-time effects
on arbitrary reaction terms (Ginn 1999). This numerical
approach supports both variable methods of accounting
of residence time (e.g., cumulative vs. contiguous) and
arbitrary specification of the effect of residence time on
the overall partitioning kinetics.
The conventional model for dilute suspended
bacteria undergoing convective-dispersive transport
and first-order kinetic reversible partitioning is, for
Hydrogeology Journal (2000) 8 : 142–158
aqueous microbes, Cmm, and attached microbes, Cim,
respectively:
iCmm
c =7(CmmV)p=7[D=Cmm]PKf CmmcKr Cim (2a)
it
iCim
p Kf CmmPKrCim
it
(2b)
where Cim is in units of biomass per aqueous volume
(pCim[biomass/solid mass]rb /u), and D, V, and the K’s
are as introduced above. This model distributes
biomass over space x and time t, so that Cmm is Cmm
(x, t). With this conventional fate-and-transport mass
balance, it is impossible to incorporate any dependence
of partitioning kinetics upon residence time, because
residence time is not in the model. In Ginn (1999), a
reformulation of the conventional fate-and-transport
mass balance is developed that allows distributions of
solutes such as biomass over space x and time t, and
generalized exposure-time (here, residence time) v on
surfaces, so that Cmm is the function Cmm(x, t, v). The
result is a mass-balance equation system just as the
above, but with the addition of a convection term
dictating the evolution of the biomass over space, time,
and the residence time coordinate v. Thus:
i(CmmV mm
iCmm
v )
c =7(CmmV) c
it
iv
p =7[D=Cmm]PKf CmmcKr(v)Cim
iCim i(CimV im
v )
c
p Kf CmmPKr(v)Cim
it
iv
(3a)
(3b)
is the rate of displacement of aqueous
where now V mm
v
biomass in the residence-time dimension, just as
is the rate of displacement in the x dimension;
VpV mm
x
and V im
v is the rate of displacement of attached biomass
in the residence-time dimension. Also, the rate of
detachment, Kr, is now expressed as a function of residence time, v, that is, KrpKr (v). If indicated, one may
also specify a dependence of attachment rate, Kf, on
residence time, v.
A subtle but important distinction exists between
the exposure-time formulation (Ginn 1999) and the
formulation presented here for residence time on
surfaces. In the original formulation, the exposure time
increases for a component whether it is in a mobile or
an immobile phase. Here, the phase association determines the residence time, with residence time
increasing only when the bacteria are on a surface
(immobile phase). Usually, the only thing known about
the attachment process is from observations at the
bulk-continuum scale, such as the effective rates of
kinetic first-order attachment and detachment.
Different attachment-detachment mechanisms may
operate and give rise to the same “bulk-scale” kinetic
first-order rate coefficients, yet these different attachment-detachment mechanisms involve very different
residence times on surfaces. For a simple illustration,
consider a detachment kinetic described by first-order
Q Springer-Verlag
153
theory with a coefficient Krp0.1 per time unit. This
means that, per time unit, 10% of the biomass is
detached and 90% of the biomass is attached at the end
of the time unit.
If the detached biomass all underwent exactly one
detachment event during that time unit, then one may
define some bulk-scale residence time of the attached
biomass. However, the newly detached bacteria do not
necessarily all undergo exactly one detachment event;
in fact, any number of bacteria may have undergone
multiple partitioning events within the time unit, as
long as the local attached and detached cell numbers
obey the postulated first-order kinetics. This nonuniqueness in basic mechanisms means that the
accounting of residence time is also non-unique and
impossible without further assumptions regarding the
underlying mechanism of attachment–detachment. The
most powerful assumption is constructed by simply
requiring the attachment-detachment process to
involve exactly one partitioning event during the time
unit specified. This assumption is basically the same
mechanistic assumption that is used to calculate the
rates of reactions with statistical thermodynamics using
transition-state theory (e.g., Kreevoy and Truhlar 1986)
and is referred to as the “no-recrossing rule.” Thus,
during one unit time, exactly Kf of the local aqueous
cells attach and exactly Kr of the local attached cells
detach, and no other partitioning events, such as an
attached cell detaching and then reattaching, occur in
that same unit time. This assumption sets the characteristic time scale of the partitioning event, and, in doing
so, links the units of the first-order rate coefficients (Kf,
Kr) with the residence time on the surface.
A rational model that is a generalization of the
Johnson et al. (1995) formulation may be written by
supposing that a limitation on detachment arises as a
result of active adhesion (e.g., via exopolysaccharide
production, biofilm formation). In this case, one may
specify a function Kr(v), where Kr (e.g., detachment
rate) decreases with increasing residence time, v. The
form of this function that is equivalent to that of
Johnson et al. (1995) is where Kr(v) is a positive
constant to some critical residence time, vpv*, beyond
which the rate of detachment [Kr(v)] is zero. That is:
Kr(v) p
Kr
50
0^v^v*
0^v*^v
(4)
In the approach of Johnson et al. (1995), an attached
cell accumulates residence time at a rate of unity per
unit time (in discrete increments), and, upon detaching,
undergoes an instantaneous decrease of residence time
to zero. This approximation reflects the notion that
cells maintain zero memory of attachment, i.e., that any
structural surface changes due to adhesion processes
are reversed upon detachment at a rate that is faster
than one discrete time interval. However, as noted in
the studies cited above, active adhesion processes are
associated with physiologic changes that occur in the
Hydrogeology Journal (2000) 8 : 142–158
microorganism on time scales that may be kinetically
controlled. For example, a newly attached microbe may
start to produce proteins and/or exopolysaccharides in
the process of biofilm formation, and if this microbe
detaches, these structures may not instantaneously
disappear. This notion is congruent with the understanding of the time scale of metabolic lag, which has
been observed on the same order as that of transport,
requiring its treatment as a kinetically controlled
process (e.g., Wood et al. 1995; Murphy et al. 1997b),
and it has recently been treated with an exposure-time
approach (Ginn 1999).
Thus it may be useful to generalize the foregoing in
order to accommodate memory or adhesion processes
for continuous residence time, v. In a general sense, the
role of memory in the attachment/detachment kinetic
rates depends on the ratio of the time scale of physiologic changes associated with active adhesion processes
to the time scale of detachment intervals, e.g., the mean
time between attachments. For illustration, consider
the two cases where kinetics of detachment depend on
(1) cumulative and (2) contiguous residence time, as
shown in Figure 4. In the cumulative memory model,
the physiological state of the microorganism depends
only on the total cumulative time that the microbe has
spent in the attached state, regardless of how that time
is distributed over attachment events or how much time
the microbe has spent in the aqueous phase
(Figure 4b). In the contiguous memory model, the
physiological state of the microorganism depends on
some finite memory of historical attachment, and so
time spent in the aqueous phase after any given attachment event may result in a kinetically controlled return
to a pre-attached state (i.e., slow loss of memory of
attachment; Figure 4c). In the cumulative case, physiological changes in the cell surface may stop if a cell
becomes detached, but they never reverse, or they
reverse so slowly that they may be considered irreversible. In the contiguous state, time spent in the aqueous
phase between attachment events can result in reversal
of the physiological changes in the cell surface.
Cumulative Case
In the case where changes in the cell surface occur so
slowly that they may be considered irreversible, then
cumulative residence time is what controls detachment
frequency. In this case, residence time needs to be
tracked during the microbes’ time spent in the solid
phase, where the rate of change in residence time, v, is
unity with time, t, i.e., the increase in residence time, v,
per unit time, t, attached is 1 : 1; thus V im
v p1. Furthermore, in this case, time spent in the aqueous phase does
nothing to the accounting of residence time, so V mm
v p
0. Thus the model becomes:
iCmm
c=7(CmmV)p=7(D7=Cmm)PKfCmmcKr(v)Cim
it
(5a)
Q Springer-Verlag
154
iCim iCim
c
p cKf CmmPKr(v)Cim
it
iv
(5b)
This case is schematically illustrated in Figure 4,
where the trajectory of a single microbe that undergoes
two attachment events during 1-D transport under a
constant velocity is illustrated first in the characteristic
plane of physical transport (x, t) (Figure 4a), and then
in the characteristic space of the model above, (x, t, v)
(Figure 4b). Physical attachment is shown in Figure 4a
at the times a (with detachment at b) and c (with irreversible residence time reached at d). The same trajectory, augmented with an explicit accounting of cumulative residence time, is shown in Figure 4b. There,
increase in the v-dimension takes place at dt:dvp1 : 1,
exactly when the microbe is attached; no increase
occurs in v when the microbe is in the aqueous phase.
With cumulative residence time, v, thus accounted, it is
possible to keep track of the time and space coordinates at which the critical cumulative residence time,
v*, is reached, and thus to keep track of the proportion
of microbes that become irreversibly attached.
Contiguous Case
In the case where cells retain some structural memory
of the changes induced by attachment but lose this
memory kinetically while in the unattached phase, it is
necessary to represent the reduction in contiguous residence time for aqueous-phase microbes (Figure 4c). In
addition to the aging velocities V im introduced in the
previous section, some non-equilibrium rate of exposure-time reduction is required in the aqueous phase
V mm
v 1 0. A simple expression for exponential (accelerated) reduction is obtained with V mm
v pPv, in which
case the model takes the form
i(vCmm)
iCmm
c =7(CmmV) P
it
iv
p =7(D7=Cmm)PKf CmmcKr(v)Cim
iCim iCim
p cKf CmmPKr(v)Cim
c
it
iv
Figure 4 a Trajectory of a single microbe undergoing transport in
a constant 1-D velocity field, with two attachment events occurring at times a and c, and indicated by horizontal portions of the
characteristic path in x, t. b Same trajectory in physical-time coordinates, now augmented with component of displacement in the
v-dimension corresponding to cumulative residence time. In this
hypothetical simplification, the microbe becomes irreversibly
attached when cumulative residence time exceeds v*. c Same
trajectory in physical-time coordinates with additional residence
time. In this contiguous case, time spent in the aqueous phase
between attachment events (b to c) results in reversal of physiological changes to cell surface that may have occurred during a
prior attachment period (a to b)
Hydrogeology Journal (2000) 8 : 142–158
(6a)
(6b)
Determination of the appropriate form for the
velocity of reduction in residence time (when it
matters) requires controlled experiments. It may also
be useful to treat this velocity as a random variable,
reflecting variability of rates of bacterial adhesion
among different individual cells. Given statistical properties of the distribution of this velocity, one might use
stochastic-analytic techniques (e.g., Gardiner 1990) to
seek the average behavior of the system.
Conclusions
Advances in modeling microbial processes in the
subsurface require a multidisciplinary approach.
Understanding the biological processes and the
Q Springer-Verlag
155
coupling of these processes with the physical flow and
transport is critical. Field and laboratory experiments
demonstrate that the metabolic activity of subsurface
microorganisms can create a dynamic distribution of a
microbial population between aqueous and solid
phases in groundwater systems. Enhanced aqueous
partitioning of the biomass can, in some cases, increase
the degradation of contaminants as a plume moves
along a groundwater gradient. In many of the examples
presented here, the complexity of the biological processes requires advances in numerical and theoretical
modeling approaches. One such advancement is the
development of an exposure-time model that allows
incorporation of cell-level processes into reactive transport models by tracking biomass in space, time, and the
additional dimension of exposure time. Using this
approach, important, distributed variables, such as residence time on a surface or the amount of time that a
microbial population has been exposed to nutrients,
can be incorporated to evaluate both the transport and
metabolic activity of a microbial population. This capability permits simulation of dynamic processes occurring in an evolving contaminant plume and is expected
ultimately to lead to a better understanding of the
subsurface behavior of microbial communities.
Acknowledgments The authors acknowledge the support of the
US Department of Energy, Office of Biological and Environmental Research, Natural and Accelerated Bioremediation
program. Also thanked are the guest editor, Barbara Bekins, and
two anonymous reviewers, who greatly improved the clarity of
this manuscript.
References
Albrechtsen H-J (1994) Distribution of bacteria, estimated by a
viable count method, and heterotrophic activity in different
size fractions of aquifer sediment. Geomicrobiol J
12 : 253–264
Bailey JE, Ollis DF (1986) Biochemical engineering fundamentals, 2nd edn. McGraw-Hill, New York
Bales RC, Hinkle SR, Kroeger TW, Stocking K (1991) Bacteriophage adsorption during transport through porous media:
chemical perturbations and reversibility. Environ Sci Technol
25 : 2088–2095
Bales RC, Li S, Maguire KM, Yahya MT, Gerba CP, Harvey RW
(1995) Virus and bacteria transport in a sandy aquifer, Cape
Cod, Massachusetts. Ground Water 33 : 653–661
Barton JW, Ford RM (1995) Determination of effective transport
coefficients for bacterial migration in sand columns. Appl
Environ Microbiol 61 : 3329–3335
Baveye P, Valocchi A (1991) Reply. Water Resour Res
27 : 1379–1380
Baveye P, Vandevivere P, Lozada DD (1992) Comment on
“Biofilm growth and the related changes in the physical properties of a porous medium, 1; experimental investigation,” by
SW Taylor and PR Jaffe. Water Resour Res 28 : 1481–1482
Beeftink HH, Heijden RTJMvd, Heijnen JJ (1990) Maintenance
requirements: energy supply from simultaneous endogenous
respiration and substrate consumption. FEMS Microbiol Ecol
73 : 203–210
Button DK (1993) Nutrient-limited microbial growth kinetics:
overview and recent advances. Antonie van Leeuwenhoek; J
Microbiol Serol 63 : 225–235
Hydrogeology Journal (2000) 8 : 142–158
Champ DR, Schroeter J (1988) Bacterial transport in fractured
rock: a field-scale tracer test at the Chalk River Nuclear Laboratories. Water Sci Technol 20 : 81–87
Chang M-K, Voice TC, Criddle CS (1993) Kinetics of competitive
inhibition and cometabolism in the biodegradation of
benzene, toluene, and p-xylene by two Pseudomonas isolates.
Biotechnol Bioeng 41 : 1057–1065
Characklis WG, Marshall KC (1990) Biofilms. John Wiley, New
York
Chen Y, Abriola LM, Alvarez PJJ, Anid PJ, Vogel TM (1992)
Modeling transport and biodegradation of benzene and
toluene in sandy aquifer material: comparison with experimental measurements. Water Resour Res 28 : 1833–1847
Chilakapati A (1995) A simulator for reactive flow and transport
of groundwater contaminants. Pacific Northwest National
Laboratory, Richland, Washington
Chilakapati A, Ginn TR, Szecsody J (1998) An analysis of
complex reaction networks in groundwater modeling. Water
Resour Res 34 : 1767–1780
Corapcioglu MY, Haridas A (1984) Transport and fate of microorganisms in porous media: a theoretical investigation. J
Hydrol 72 : 149–169
Corapcioglu MY, Kim S (1996) Modeling facilitated contaminant
transport by mobile bacteria. Water Resour Res
31 : 2639–2647
Criddle CS (1993) The kinetics of cometabolism. Biotechnol
Bioeng 41 : 1048–1056
Dawson MP, Humphrey A, Marshall KC (1981) Adhesion: a
tactic in the survival strategy of a marine vibrio during starvation. Curr Microbiol 6 : 195–199
de Marsily G (1986) Quantitative hydrogeology. Academic Press,
New York
Dodds J (1982) La chromatographie hydrodynamique. Analusis
10 : 109–119
Eisenmann H, Harms H, Meckenstock R, Meyer EI, Zehnder
AJB (1998) Grazing of a Tetrahymena sp. on adhered bacteria
in percolated columns monitored by in situ hybridization with
fluorescent oligonucleotide probes. Appl Environ Microbiol
64 : 1264–1269
Ely RL, Williamson KJ, Hyman MR, Arp DJ (1997) Cometabolism of chlorinated solvents by nitrifying bacteria: kinetics, substrate interactions, toxicity effects, and bacterial
response. Biotechnol Bioeng 54 : 520–534
Enfield CG, Bengtsson G (1988) Macromolecular transport of
hydrophobic contaminants in aqueous environments. Ground
Water 26 : 64–70
Fletcher M (1991) Bacterial colonization of solid surfaces in
subsurface environments. In: Fliermans CB, Hazen TC (eds)
Proc 1st Int Symp on Microbiology of the Deep Subsurface,
Orlando, Florida, 1990
Fletcher M (1996) Bacterial adhesion: molecular and ecological
diversity. In: Mitchell R (ed) Ecological and applied microbiology. Wiley-Liss, New York, 361 pp
Fletcher M, Loeb GI (1979) Influence of substratum characteristics on the attachment of a marine pseudomonad to solid
surfaces. Appl Environ Microbiol 37 : 67–72
Ford RM, Cummings PT (1998) Mathematical models of bacterial
chemotaxis. In: Koch AL, Robinson JA, Milliken GA (eds)
Mathematical modeling in microbial ecology. Microbiology
Series. Chapman & Hall, New York, 273 pp
Gardiner CW (1990) Handbook of stochastic methods for
physics, chemistry, and the natural sciences. Springer, Berlin
Heidelberg New York
Gilbert P, Brown MRW (1995) Mechanisms of the protection of
bacterial biofilms from antimicrobial agents. In: Lappin-Scott
HM, Costerton JW (eds) Microbial biofilms. Cambridge
University Press, Cambridge, pp 118–130
Ginn TR (1999) On the distribution of multi-component mixtures
over generalized exposure-time in subsurface flow and reactive transport: foundations, and formulations for groundwater
age, chemical heterogeneity, and biodegradation. Water
Resour Res 35 : 1395–1408
Q Springer-Verlag
156
Ginn TR, Simmons CS, Wood BD (1995) Stochastic-convective
transport with nonlinear reaction: biodegradation with microbial growth. Water Resour Res 31 : 2689–2700
Ginn TR, Scheibe TD, Murphy EM, DeFlaun MF, Onstott TC
(1998) Effects of chemical heterogeneity on subsurface fate
and transport involving biotic reaction systems: two examples.
In: American Geophysical Union Fall Meeting, San Francisco,
December 1998. Eos Trans 79(45):F294
Godsy EM, Goerlitz DF, Grbic-Galic D (1992) Methanogenic
biodegradation of creosote contaminants in natural and simulated ground-water ecosystems. Ground Water 30 : 232–242
Harms H, Zehnder AJB (1994) Influence of substrate diffusion
on degradation of dibenzofuran and 3-chlorodibenzofuram by
attached and suspended bacteria. Appl Environ Microbiol
60 : 2736–2745
Harvey RW (1991) Parameters involved in modeling movement
of bacteria in groundwater. In: Hurst CJ (ed) Modeling the
environmental fate of microorganisms. American Society for
Microbiology, Washington, DC, pp 89–114
Harvey RW, Barber LB (1992) Associations of free-living
bacteria and dissolved organic compounds in a plume of
contaminated groundwater. J Contam Hydrol 9 : 91–103
Harvey RW, Garabedian SP (1991) Use of colloid filtration
theory in modeling movement of bacteria through a contaminated sandy aquifer. Environ Sci Technol 25 : 178–185
Harvey RW, Widdowson MA (1992) Microbial distributions,
activities, and movement in the terrestrial subsurface: experimental and theoretical studies. In: Wagenet RJ, Baveye P,
Stewart BA (eds) Advances in soil science. Lewis Publishers,
Boca Raton, pp 185–225
Harvey RW, Smith RL, George L (1984) Effect of organic
contamination upon microbial distributions and heterotrophic
uptake in a Cape Cod, Mass., aquifer. Appl Environ Microbiol
48 : 1197–1202
Harvey RW, George LH, Smith RL, LeBlanc DR (1989) Transport of microspheres and indigenous bacteria through a sandy
aquifer: results of natural- and forced-gradient tracer experiments. Environ Sci Technol 23 : 51–56
Harvey RW, Kinner NE, MacDonald D, Metge DW, Bunn A
(1993) Role of physical heterogeneity in the interpretation of
small-scale laboratory and field observations of bacteria,
microbial-sized microsphere, and bromide transport through
aquifer sediments. Water Resour Res 29 : 2713–2721
Harvey RW, Kinner NE, Bunn A, MacDonald D, Metge D
(1995) Transport behavior of groundwater protozoa and
protozoan-sized microspheres in sandy aquifer sediments.
Appl Environ Microbiol 61 : 209–217
Harvey RW, Metge DW, Kinner N, Mayberry N (1997) Physiological considerations in applying laboratory-determined
buoyant densities to predictions of bacterial and protozoan
transport in groundwater: results of in-situ and laboratory
tests. Environ Sci Technol 31 : 289–295
Herbert D (1958) Some principles of continuous culture. In:
Tunevall G (ed) Recent progress in microbiology. Blackwell
Scientific, Oxford, pp 381–396
Herzig JP, Leclerc DM, LeGoff P (1970) Flow of suspension
through porous media; application to deep filtration. Ind Eng
Chem 62 : 129–157
Hess TF, Schmidt SK, Colores GM (1996) Maintenance energy
model for microbial degradation on toxic chemicals in soil.
Soil Biol Biochem 28 : 907–915
Hirsch P, Rades-Rohkohl E (1988) Die Vielfalt mikrobieller
Mosrphotypen im Grundwasservereich des Segeberger
Forstes. Z Dtsch Geol Ges 139 : 343–353
Hornberger GM, Mills AL, Herman JS (1992) Bacterial transport
in porous media: evaluation of a model using laboratory
observations. Water Resour Res 28 : 915–938
Jaffe PR, Taylor SW (1992) Reply. Water Resour Res
28 : 1483–1484
Jenneman GE, McInerney MJ, Knapp RM (1985) Microbial
penetration through nutrient-saturated Berea sandstone. Appl
Environ Microbiol 50 : 383–391
Hydrogeology Journal (2000) 8 : 142–158
Jenneman GE, McInerney MJ, Crocker MF, Knapp RM (1986)
Effect of sterilization by dry heat or autoclaving on bacterial
penetration through Berea sandstone. Appl Environ Microbiol 51 : 39–43
Jewett DG, Hilbert TA, Logan BE, Arnold RG, Bales RC (1995)
Bacterial transport in laboratory columns and filters: influence
of ionic strength and pH on collision efficiency. Water Res
29 : 1673–1680
Johnson WP, Blue KA, Logan BE, Arnold RG (1995) Modeling
bacterial detachment during transport through porous media
as a residence-time-dependent process. Water Resour Res
31 : 2649–2658
Jucker BA, Harms H, Hug SJ, Zehnder AJB (1997) Adsorption
of bacterial surface polysaccharides on mineral oxides is
mediated by hydrogen bonds. Series B: Biointerfaces. Colloids
Surfaces 9 : 331–343
Kaprelyants AS, Gottschal JC, Kell DB (1993) Dormancy in nonsporulating bacteria. FEMS Microbiol Rev 104 : 271–286
Kindred JS, Celia MA (1989) Contaminant transport and biodegradation, 2. Conceptual model and test simulations. Water
Resour Res 1989 : 1149–1159
Kinoshita T, Bales RC, Yahya MT, Gerba CP (1993) Bacteria
transport in a porous medium: retention of bacillus and pseudomonas on silica surfaces. Water Res 27 : 1295–1301
Kinzelbach W, Schafer W, Herzer J (1991) Numerical modeling
of natural and enhanced denitrification processes in aquifers.
Water Resour Res 27 : 1123–1135
Kjelleberg S, Hermansson M (1984) Starvation-induced effects on
bacterial surface characteristics. Appl Environ Microbiol
48 : 497–503
Kjelleberg S, Humphrey BA, Marshall KC (1982) The effect of
interfaces on small starved marine bacteria. Appl Environ
Microbiol 43 : 1166–1172
Kjelleberg S, Hermansson M, Marden P (1987) The transient
phase between growth and nongrowth of heterotrophic
bacteria, with emphasis on the marine environment. Annu
Rev Microbiol 41 : 25–49
Koch AL (1998) The Monod model and its alternatives. In: Koch
AL, Robinson JA, Milliken GA (eds) Mathematical modeling
in microbial ecology. Microbiology Series. Chapman & Hall,
New York, pp 62–93
Kölbel-Boelke J, Anders E, Nehrkorn A (1988) Microbial
communities in the saturated groundwater environment II:
diversity of bacterial communities in a Pleistocene sand
aquifer and their in vitro activities. Microb Ecol 16 : 31–48
Kreevoy MM, Truhlar DG (1986) Transition state theory. In:
Bernasconi CF (ed) Investigations of rates and mechanisms of
reactions, Part 1. General considerations and reactions at
conventional rates, 4th edn. Wiley, New York, pp 13–97
Lang MM, Roberts PV, Semprini L (1997) Model simulations in
support of field scale design and operation of bioremediation
based on cometabolic degradation. Ground Water
35 : 565–573
Lindqvist R, Bengtsson G (1991) Dispersal dynamics of groundwater bacteria. Microb Ecol 21 : 49–72
Lindqvist R, Cho JS, Enfield CG (1994) A kinetic model for cell
density dependent bacterial transport in porous media. Water
Resour Res 30 : 3291–3299
Little CD, Palumbo AV, Herbes SE, Lidstrom ME, Tyndall RL,
Gilmer PJ (1988) Trichloroethylene biodegradation by a
methane-oxidizing bacterium. Appl Environ Microbiol
54 : 951–956
Logan BE, Jewett DG, Arnold RG, Bouwer EJ, O’Melia CR
(1995) Clarification of clean-bed filtration models. J Environ
Eng December:869–873.
MacQuarrie KTB, Sudicky EA, Frind EO (1990) Simulation of
biodegradable organic contaminants in groundwater. 1.
Numerical formulation in principal directions. Water Resour
Res 26 : 207–222
Marshall KC (1996) Adhesion as a strategy for access to
nutrients. In: Fletcher M (ed) Bacterial adhesion, molecular
and ecological diversity. John Wiley, New York, pp 59–87
Q Springer-Verlag
157
Matin A (1990) Molecular analysis of the starvation stress in E.
coli. FEMS Microbiol Ecol 74 : 185–196
Mayotte TJ, Dybas MJ, Criddle CS (1996) Bench-scale evaluation
of bioaugmentation to remediate carbon tetrachloride
contaminated aquifer materials. Ground Water 34 : 358–367
McCaulou DR, Bales RC, McCarthy JF (1994) Use of short-pulse
experiments to study bacteria transport through porous
media. J Contam Hydrol 15 : 1–14
McCaulou DR, Bales RC, Arnold RG (1995) Effect of temperature-controlled motility on transport of bacteria and microspheres through saturated sediment. Water Resour Res
31 : 271–280
McDowell-Boyer LM (1992) Chemical mobilization of micronsized particles in saturated porous media under steady flow
conditions. Environ Sci Technol 26 : 586–593
McDowell-Boyer LM, Hunt JR, Sitar N (1986) Particle transport
through porous media. Water Resour Res 22 : 1901–1921
McEldowney S, Fletcher M (1988) Effect of pH, temperature, and
growth conditions on the adhesion of a gliding bacterium and
three nongliding bacteria to polystyrene. Microb Ecol
16 : 183–195
Mercer JR, Ford RM, Stitz JL, Bradbeer C (1993) Growth rate
effects on fundamental transport properties of bacterial populations. Biotechnol Bioeng 42 : 1277–1286
Mills WB, Liu S, Fong FK (1991) Literature review and model
(comet) for colloid/metals transport in porous media. Ground
Water 29 : 199–208
Mills AL, Herman JS, Hornberger GM, de Jesus TH (1994)
Effect of ionic strength and iron coatings on mineral grains on
sorption of bacterial cells to quartz sand. Appl Environ Microbiol 60 : 3300–3306
Mohn WW, Tiedje JM (1992) Microbial reductive dehalogenation. Microbiol Rev 56 : 482–507
Molz FJ, Widdowson MA, Benefield LD (1986) Simulation of
microbial growth dynamics coupled to nutrient and oxygen
transport in porous media. Water Resour Res 22 : 1207–1216
Monod J (1949) The growth of bacterial cultures. Annu Rev
Microbiol 3 : 371–393
Murphy EM, Ginn TR, Brockman FJ, Boone DR (1997a) Growth
effects on the partitioning and transport of bacteria. In: American Geophysical Union Fall Meeting, San Francisco,
December 1997. Eos Trans 78(46) : F231
Murphy EM, Ginn TR, Chilakapati A, Resch CT, Phillips JL,
Wietsma TW, Spadoni CM (1997b) The influence of physical
heterogeneity on microbial degradation and distribution in
porous media. Water Resour Res 33 : 1087–1103
Novitsky JA, Morita RY (1976) Morphological characterization
of small cells resulting from nutrient starvation in a psychrophilic marine vibrio. Appl Environ Microbiol 32 : 619–622
Novitsky JA, Morita RY (1977) Survival of a psychrophilic
marine vibrio under long-term nutrient starvation. Appl
Environ Microbiol 33 : 635–641
Oliver J, Nilsson L, Kjelleberg S (1991) Formation of nonculturable Vibrio vulnificus cells and its relationship to the starvation state. Appl Environ Microbiol 57 : 2640–2644
Petzold L (1983) A description of DASSL: a differential/algebraic
system solver. In: Stepelman RS (ed) Scientific computing:
applications of mathematics and computing to the physical
sciences. North Holland, New York, pp 65–68
Pirt SJ (1975) Principles of microbe and cell cultivation. John
Wiley, New York
Powell EO (1967) The growth rate of microorganisms as a function of the substrate concentration. In: Powell EO, Evans GT,
Strange RE, Tempest DW (eds) Microbial physiology and
continuous culture. HMSO, London, pp 34–56
Pyle BH (1979) Technical publication no 2. Lincoln College
Department of Agriculture Microbiology, Canterbury, New
Zealand
Pyle BH, Thorpe HR (1981) Evaluation of the potential for
microbiological contamination of an aquifer using a bacterial
tracer. In: Proc Conf on Ground-Water Pollution, Sydney.
Australian Water Resources Council Conference Series
1 : 213–224
Hydrogeology Journal (2000) 8 : 142–158
Rajagopalan R, Tien C (1976) Trajectory analysis of deep-bed
filtration with the sphere-in-a-cell porous media model. Am
Inst Chem Eng 22 : 523–533
Reynolds PJ, Sharma P, Jenneman GE, McInerney MJ (1989)
Mechanisms of microbial movement in subsurface materials.
Appl Environ Microbiol 55 : 2280–2286
Rijnaarts HHM, Norde W, Bouwer EJ, Lyklema J, Zehnder AJB
(1993) Bacterial adhesion under static and dynamic conditions. Appl Environ Microbiol 59 : 3255–3265
Rittman BE (1993) The significance of biofilms in porous media.
Water Resour Res 29 : 2195–2202
Saiers JE, Hornberger GM (1996) The role of colloidal kaolinite
in the transport of cesium through laboratory sand columns.
Water Resour Res 32 : 33–41
Sakthivadivel R (1966) Theory and mechanism of filtration of
non-colloidal fines through a porous medium. Report HEL
15-5. Hydraulic Engineering Laboratory, University of California, Berkeley, 110 pp
Sakthivadivel R (1969) Clogging of a granular porous medium by
sediment. Report HEL 15-7. Hydraulic Engineering Laboratory, University of California, Berkeley, 106 pp
Scholl MA, Mills AL, Herman JS, Hornberger GM (1990) The
influence of mineralogy and solution chemistry on the attachment of bacteria to representative aquifer materials. J Contam
Hydrol 6 : 321–336
Semprini L, McCarty PL (1992) Comparison between model
simulations and field results for in-situ biorestoration of chlorinated aliphatics: Part 2. Cometabolic transformations.
Ground Water 30 : 37–44
Semprini L, Hopkins GD, Roberts PV, Grbic-Galic D, McCarty
PL (1991) A field evaluation of in-situ biodegradation of chlorinated ethenes: Part 3. Studies of competitive inhibition.
Ground Water 29 : 239–250
Sharma MM, Chang YI, Yen TF (1985) Reversible and irreversible surface charge modifications of bacteria for facilitating
transport through porous media. Colloids Surfaces
16 : 193–206
Sharma PK, McInerney MJ, Knapp RM (1993) In situ growth and
activity and modes of penetration of Escherichia coli in unconsolidated porous materials. Appl Environ Microbiol
59 : 3686–3694
Shaw DJ (1970) Introduction to colloid and surface chemistry,
2nd edn. Butterworth-Heinemann, Woburn, Massachusetts,
pp 168–177
Shonnard DR, Taylor RT, Hanna ML, Boro CO, Duba AG
(1994) Injection-attachment of Methylosinus trichosporium
OB3b in a two-dimensional miniature sand-filled aquifer
simulator. Water Resour Res 30 : 25–36
Smigielski A, Wallace B, Marshall K (1989) Changes in
membrane functions during short-term starvation of Vibrio
fluvialis strain NCTC 11328. Arch Microbiol 151 : 336–341
Smith JL (1989) Sensitivity analysis of critical parameters in
microbial maintenance-energy models. Biol Fert Soils 8 : 7–12
Smith LH, McCarty PL (1997) Laboratory evaluation of a twostage treatment system for TCE cometabolism by a methaneoxidizing mixed culture. Biotechnol Bioeng 55 : 650–659
Smith JL, McNeal BL, Cheng HH, Campbell GS (1986) Calculation of microbial maintenance rates and net nitrogen mineralization in soil at steady-state. Soil Sci Soc Am J 50 : 332–338
Smith LH, Kitanidis PK, McCarty PL (1997) Numerical modeling
and uncertainties in rate coefficients for methane utilization
and TCE cometabolism by a methane-oxidizing mixed culture.
Biotechnol Bioeng 53 : 320–331
Steefel CI, MacQuarrie KTB (1996) Approaches to modeling of
reactive transport in porous media. In: Lichtner PC, Steefel
CI, Oelkers EH (eds) Reactive transport in porous media.
Mineral Soc Am Rev Mineral 34 : 83–130
Sudicky EA, Schellenberg SL, MacQuarrie KTB (1990) Assessment of the behaviour of conservative and biodegradable
solutes in heterogeneous porous media. In: Cushman JH (ed)
Dynamics of fluids in hierarchical porous media. Academic
Press, San Diego, pp 429–462
Q Springer-Verlag
158
Tan Y, Gannon JT, Baveye P, Alexander M (1994) Transport of
bacteria in an aquifer sand: experiments and model simulations. Water Resour Res 30 : 3243–3252
Taylor SW, Jaffe PR (1990) Substrate and biomass transport in a
porous medium. Water Resour Res 26 : 2181–2194
Taylor SW, Milly PCD, Jaffe PR (1990) Biofilm growth and
related changes in the physical properties of a porous medium
2. Permeability. Water Resour Res 26 : 2161–2170
Tien C, Turian RM, Pandse H (1979) Simulation of the dynamics
of deep bed filters. Am Inst Chem Eng 25 : 385–395
USDOE (U.S. Department of Energy) (1993) Cleanup of VOCs
in non-arid soils – the Savannah River integrated demonstration. US Department of Energy, Environmental Restoration
and Waste Management Office of Technology Development,
Washington, DC
Vandevivere P, Baveye P (1992) Saturated hydraulic conductivity
reduction caused by aerobic bacteria in sand columns. J Soil
Sci Soc Am 56 : 1–13
van Loosdrecht MCM, Lyklema J, Norde W, Schraa G, Zehnder
AJB (1987) Electrophoretic mobility and hydrophobicity as a
measure to predict the initial steps of bacterial adhesion. Appl
Environ Microbiol 53 : 1898–1901
van Loosdrecht MCM, Lyklema J, Norde W, Zehnder AJB
(1989) Bacterial adhesion: a physiochemical approach. Microb
Ecol 17 : 1–15
van Loosdrecht MCM, Lyklema J, Norde W, Zehnder AJB
(1990) Influence of interfaces on microbial activity. Microbiol
Rev 54 : 75–87
Wan J, Tokunaga TK, Tsang CF (1995) Bacterial sedimentation
through a porous medium. Water Resour Res 31 : 1627–1636
Widdowson MA (1991) Comment on “An evaulation of mathematical models of the transport of biologically reacting solutes
in saturated soils and aquifers.” Water Resour Res
27 : 1375–1378
Hydrogeology Journal (2000) 8 : 142–158
Widdowson MA, Molz FJ, Benefield LD (1988) A numerical
transport model for oxygen- and nitrate-based respiration
linked to substrate and nutrient availability in porous media.
Water Resour Res 24 : 1553–1565
Williams V, Fletcher M (1996) Adhesion and transport through
porous media of Pseudomonas fluorescens is affected by lipopolysaccharide composition. Appl Environ Microbiol
62 : 100–104
Wood WW, Ehrlich GG (1978) Use of baker’s yeast to trace
microbial movement in ground water. Ground Water
16 : 398–403
Wood BD, Whitaker S (1998) Diffusion and reaction in biofilms.
Chem Eng Sci 53 : 397–425
Wood BD, Whitaker S (1999) Cellular growth in biofilms.
Biotechnol Bioeng 64 : 656–670
Wood BD, Whitaker S (in press) Multispecies diffusion and reaction in biofilms. Chem Eng Sci
Wood BD, Dawson CN, Szecsody JE, Streile GP (1994) Modeling
contaminant transport and biodegradation in a layered porous
media system. Water Resour Res 30 : 1833–1845
Wood BD, Ginn TR, Dawson CN (1995) Effects of microbial
metabolic lag in contaminant transport and biodegradation
modeling. Water Resour Res 31 : 553–563
Wrangstadh M, Szewzyk U, Oestling J, Kjelleberg S (1990) Starvation-specific formation of a peripheral exopolysaccharide by
a marine Pseudomonas sp., strain S9. Appl Environ Microbiol
56 : 2065–2072
Yeh G, Tripathi V (1989) A critical evaluation of recent developments in hydrogeochemical transport models of reactive
multichemical components. Water Resour Res 25 : 93–108
Zysset A, Stauffer F, Dracos T (1994) Modeling of reactive
groundwater transport governed by biodegradation. Water
Resour Res 30 : 2423–2434
Q Springer-Verlag